EDCTP COVID-19 emergency funding for twenty research projects
The COVID-19 pandemic led to an unprecedented surge in scientific collaboration on research and development programs for prevention, diagnostic and therapeutic tools. In April 2020, EDCTP contributed to this effort by activating its Emergency funding mechanism to ensure a quick start of COVID-19 research in sub-Saharan Africa.
The COVID-19 Emergency response call was open for only 14 days and saw a record number of applications submitted to a single-stage call with an accelerated review mechanism. A total of 153 applications were received, of which 100 were eligible. The consortia which were assembled in a couple of days to respond to the call and -the high quality of applications received may be an indicator of growing strength and competitiveness of the clinical research community in sub-Saharan Africa which is further bolstered by international collaboration.
The 100 eligible applications went through an expedited and robust peer review which led to a ranked list of 47 proposals. Twenty proposals have been invited to grant preparation and some put on a reserve list while EDCTP continues to explore ways to fund these projects. The cash contribution from the European Union and the co-funding so far received so from France, Sweden, South Africa, and the United Kingdom is gratefully acknowledged.
“Our quick activation of the Emergency mechanism with substantial contributions of several member countries is a testimony of EDCTP’s determination to support the battle against the pandemic. We have been energised by the overwhelming response to the call and the dedication of the experts who made our expedited review procedure possible.”
Dr Michael Makanga, EDCTP Executive Director
Analysis of the selected proposals
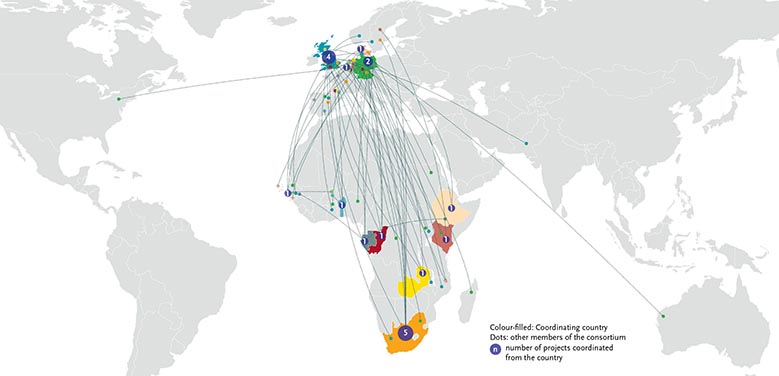
The 20 selected projects will be conducted by 20 consortia (each with at least 3 member institutions). Twelve consortia are coordinated by entities based in sub-Saharan Africa.
Map: 20 projects, 20 Networks (12 led by SSA institutions) (click to enlarge)
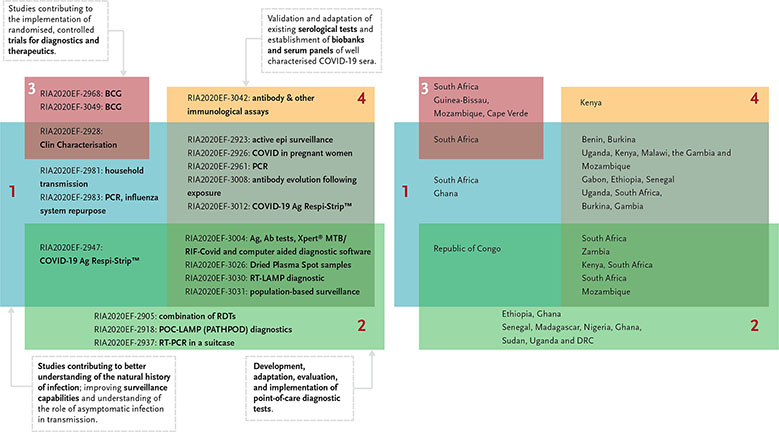
The call had invited proposals that would focus on the following thematic areas while aiming to:
- Understand the natural history of infection and the role of asymptomatic infection in transmission, improve surveillance capabilities.
- Promote the development, adaptation, evaluation, and implementation of point-of-care diagnostic tests that can be used to screen all patients presenting with a history consistent with COVID-19 infection.
- Support standardised implementation of randomised, controlled trials of promising products (diagnostics and therapeutics).
- Validate and adapt existing serological tests and establish biobanks and serum panels of well-characterised COVID-19 sera.
The EDCTP Project Officer for the call, Jean Marie Vianney Habarugira, did a first analysis of the 20 selected projects. It shows that most proposals are seeking to address more than one thematic area and involve different countries across sub-Saharan Africa.
Description of the objectives of the selected proposals
- The RE-BCG-CoV-19 project (RIA2020EF-2968) aims to investigate whether and why BCG-revaccination can reduce infection rate and/or disease severity in healthcare workers during the SARS-CoV-2 virus disease outbreak in South Africa.
- The BCG-COVID-RCT project (RIA2020EF-3049) aims to study the BCG non-specific protection of healthcare workers against COVID-19.
- The HALT_COVID-19 project (RIA2020EF-2928) aims to utilize a combination of approaches to characterise clinical disease progression and clinical outcomes among patients with SARS-CoV-2 viral infection and to perform a clinical evaluation of COVID-19 testing kits in South Africa.
- The Covid-19 HCW project (RIA2020EF-3020) aims to investigate the epidemiology of SARS-CoV-2 infection among (i) healthcare workers who triage patients with suspected SARS-CoV-2 infection and provide care to COVID-19 patients, and ii) laboratory personnel who test clinical samples for SARS-CoV-2 infection.
- The TraCE project (RIA2020EF-2981) aims to understand and mitigate household transmission of SARs-CoV2 infection in a low-income, high-density South African community setting. Data to be collected over 8 months will be used to determine the Ro for SARS-CoV-2 infection, the rate of symptomatic disease, and the impact of an infection mitigation intervention administered by community healthcare workers.
- The CSIGN project (RIA2020EF-2983) aims to build the Ghana COVID-19 surveillance and response system, by amplifying and redirecting the already excellent influenza surveillance toward the new pathogen, by enhancing response activities through contact tracing, and by building capacity in laboratory testing and sequencing.
- The ITAIL-COVID-19 project (RIA2020EF-2947) aims to better understand the COVID-19 infection epidemiology in Congo-Brazzaville and to strengthen the country’s national surveillance system. Surveillance will be carried out by assessing the circulation of the virus in the community, in various ways (patients with flu-like symptoms and a symptom-free control group).
- The Profile-Cov project (RIA2020EF-2905) aims to evaluate the profile of the immune response of the Ethiopian population and will examine its relationship with the noted low CD4+ T-cell count and underlying immune activation status among patients with COVID-19 and will compare the results with data collected in Europe.
- The AfriDx project (RIA2020EF-2918) aims to conduct a trial of a prototype point-of-care LAMP test (PATHPOD) for detection of viral RNA (SARS-Cov-2) in the COVID-19 testing laboratories in Ghana; develop a production process for local manufacture in Ghana of the required biological materials at low cost; produce a version of PATHPOD in Africa for Africa (the AfriDx).
- The Africa_Suitcaslab project (RIA2020EF-2937) aims to validate a solar-powered suitcase RT PCR lab for the detection of SARS-COV-2. Conducted in seven African countries, the study will include capacity building by providing a BSL-3 level glove box to allow safe inactivation of the clinical samples as well as strengthening the diagnostic capabilities of the involved partners.
- The ImmunoCoV project (RIA2020EF-3042) aims to 1) test and validate antibody and other immunological assays for the diagnosis and further research on the immunology of SARS-CoV-2 infections in African populations, and 2) provide detailed kinetics of the immune response to SARS-CoV-2 in relation to viral shedding, clinical symptoms and asymptomatic infections.
- The STREESCO project (RIA2020EF-2923) aims to develop a system for epidemic surveillance and response that will be effective, sensitive, coordinated and adapted to the low-resource context in Benin and Burkina Faso.
- The periCOVID-Africa project (RIA2020EF-2926) aims to develop COVID-19 surveillance in pregnancy in The Gambia, Kenya, Malawi, Mozambique and Uganda embedded in existing cohort studies.
- The AIDCO project (RIA2020EF-2961) aims to characterise COVID-19 in different parts of Africa, studying the clinical outcome of COVID-19 and the pattern of infection in households with confirmed cases in Senegal, Gabon and Ethiopian.
- The COVAB project (RIA2020EF-3008) aims to understand antibody evolution following exposure to and/or SARS-CoV-2 infection, and to investigate factors associated with infection by SARS-CoV-2 across oral and nasal mucosa biopsies.
- The COVADIS project (RIA2020EF-3012) aims to validate an antigen-based point of care test in suspected West African COVID-19 patients; to develop a validated serological platform for COVID-19 serodiagnostic and outbreak surveillance, to characterise the viral load and antibody dynamics in a cohort of confirmed COVID-19 patients and to characterise at the household level the seroepidemiology of COVID-19 infections.
- The TREATS-COVID project (RIA2020EF-3004) aims to conduct epidemiological and social research that will improve our understanding of SARS-CoV-2 transmissibility, susceptibility, disease severity, and risk factors and the interrelationship between SARS-CoV-2 and TB/HIV.
- The COREP project (RIA2020EF-3026) aims to 1) define epidemiological parameters of SARS-CoV-2 infection in rural Kenya and South Africa, including the reproductive number, transmissibility, clinical disease spectrum and population-level incidence; 2) assess the burden of SARS-CoV-2 disease in rural areas of sub-Saharan Africa, and risk factors for infection and transmission; and 3) evaluate the diagnostic performance, feasibility, usability and cost-effectiveness of Dried Plasma Spot samples collected at the community level through fingerpricks by community health workers, followed by centralised serological testing.
- The RADIATES Consortium (RIA2020EF-3030) aims to optimise and evaluate a cost-effective reverse transcriptional loop-mediated isothermal amplification (RT-LAMP) diagnostic platform for point-of-care testing in resource-limited settings.
- The AfriCoVER project (RIA2020EF-3031) aims to conduct population-based surveillance in a Demographic Health Surveillance System (HDSS) of 16,500 people in a peri-urban neighbourhood of Maputo, Mozambique, by collecting clinical information and respiratory specimens from patients during biweekly household visits, complemented with data from local clinics.