European Parliament approves second EDCTP programme
Today, the European Parliament has approved the participation of the European Union in the second programme of the European & Developing Countries Clinical Trials Partnership (EDCTP). On 6 May, the Council will vote on the proposal. With a budget of € 683 million from the European Union to be matched by the participating Member States, the programme will support all stages of clinical trials, from phase I to IV, on HIV/AIDS, tuberculosis, malaria and neglected infectious diseases.
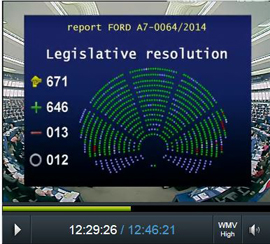
““We are very excited with the overwhelming support from the European Parliament. We are now anxiously looking forward to the start of the second programme of EDCTP after the European Council has voted on the proposal.”
Prof. Charles Mgone, EDCTP Executive Director
EDCTP Association
As part of the preparations for the second programme, EDCTP has changed its legal structure for the second programme from European Economic Interest Grouping (EEIG) to an international Association under the Dutch law. This will enable Horizon 2020-associated countries as well as sub-Saharan member states to become full members.
The EDCTP Association was established on 10 April 2014 in The Hague, the Netherlands. Representatives of the United Kingdom and France signed the incorporation papers as the dedicated implementation structure of the programme.

The other EDCTP Participating States, including the African countries that have agreed to join the programme, will be invited to officialise their membership at the EDCTP General Assembly meeting in May. Eight new countries have agreed to join the EDCTP-Association so far: Finland and the Republics of Cameroon, Congo, Mozambique, Senegal, South Africa, Uganda and Zambia.
Scope of EDCTP2
The second EDCTP programme will continue to support the clinical development of new or improved diagnostics, drugs, vaccines and microbicides against HIV/AIDS, tuberculosis and malaria. In addition, EDCTP will include support to studies on neglected infectious diseases (NIDs) as defined in the WHO list of 17 NIDs (except Chagas disease). All stages of clinical trials will be supported, from phase I to IV, though the main focus will be on phase II and III clinical trials. The geographical focus of EDCTP2’s activities will remain on sub-Saharan Africa, although collaborative research with other developing countries outside sub-Saharan Africa could be envisioned when possible and desirable.
EDCTP will continue to promote and support: multicentre projects that combine clinical trials, capacity building and networking; capacity development for clinical trials and clinical research in sub-Saharan countries; closer collaboration with industry, like-minded product development partners and funding or development agencies.
Workplans 2014-2015
EDCTP is in the process of preparing a workplan for the first two years of the programme. This first workplan will cover the initial two years of the second EDCTP programme (2014-2015), in order to align with the EC work programmes for Horizon 2020. In subsequent years the workplan will be published annually, covering one-year periods. It is anticipated that the Calls for Proposals for 2014-2015 will include broad topics in clinical research as well as various types of fellowships.
More information
Updated 20 May 2014
Final decision of the European Parliament and the Council on EDCTP2
