International Clinical Trials Day 2017: trials and results
Ahead of International Clinical Trials Awareness Day 2017, an important joint statement was released by international funders of clinical research to further protect the integrity and enhance the impact of clinical trials.
On 18 May 2017, the first signatories pledged to implement within 12 months “policies for mandated prospective registration and public disclosure of the results of clinical trials that we fund, co-fund, sponsor or support”. They affirm that this is of “critical scientific and ethical importance”. “Furthermore, timely results disclosure reduces waste in research, increases value and efficiency in use of funds and reduces reporting bias, which should lead to better decision-making in health.” EDCTP fully adheres to these views and intends to join this initiative as a signatory.
“On 4 May 2017, the EDCTP Association Board endorsed the joint statement to implement WHO standards on reporting clinical trial results. As we fund highly collaborative research, we believe the public disclosure of results will be of mutual benefit to all individuals and organisations who make clinical research possible.”
Dr Michael Makanga, EDCTP Executive Director
The need for clinical trials
Clinical trials play a critical role in product development for use in the fight against disease. The international Clinical Trials awareness day serves to celebrate the life-saving achievements of clinical research, discuss the best way to conduct trials, and extend our thanks to the participating volunteers. EDCTP funds clinical research conducted in sub-Saharan Africa in order to help address its greatest unmet needs regarding infectious diseases related to poverty.
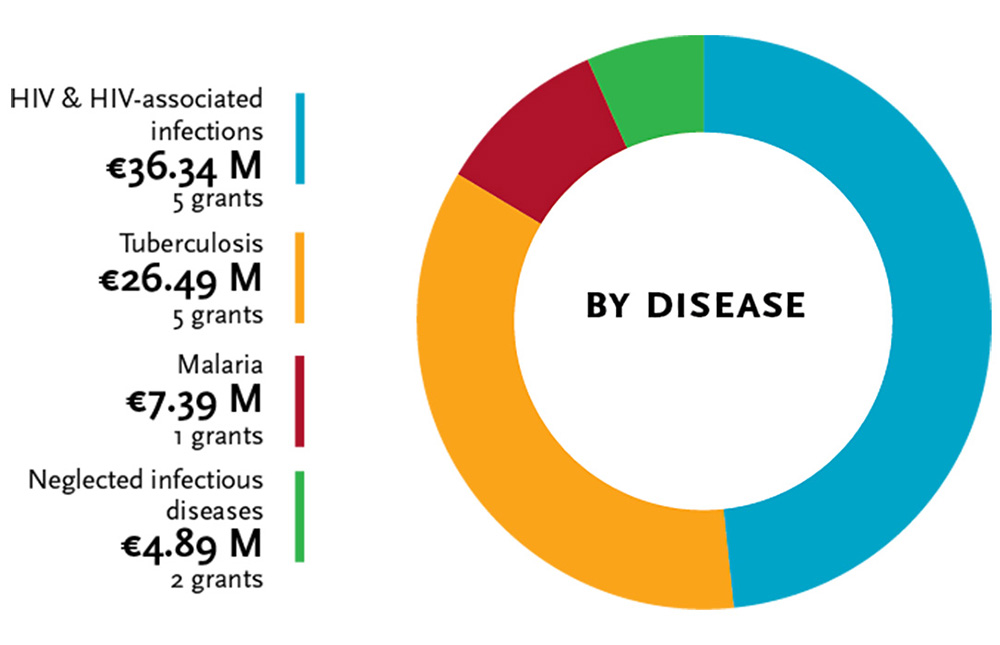
Under its first programme EDCTP (2003-2014) funded 102 clinical trials across its scope to a total amount of €176.27 million. These clinical studies were on drugs, vaccines, microbicides and diagnostics regarding HIV, tuberculosis and malaria.
The second EDCTP (2014-2024) programme has a broader scope. It encompasses in addition to HIV, tuberculosis and malaria, the neglected infectious diseases, lower respiratory tract infections, diarrhoeal diseases, as well as emerging and re-emerging infectious diseases relevant for sub-Saharan Africa. The calls for proposals from the first years (2014-2016) resulted thus far in 13 grants for large clinical research studies (Research and Innovation Actions) with a total value of €75.11 million.
An overview of the currently funded projects is available on the website. Large clinical research studies which have recently started are:
- the GREAT project, an African-European partnership to evaluate new HIV vaccines;
- the CHAPAS-4 clinical trial which will evaluate second-line treatment of children with HIV-infection;
- the PanACEA2 clinical trials on tuberculosis treatment shortening;
- the Predict-TB project on biomarkers to predict TB treatment duration;
- the AMBITION clinical trial which studies cryptococcal meningitis treatment; and
- the IMPROVE clinical trial to evaluate alternative treatment for malaria, sexually transmitted and reproductive tract infections in pregnancy in view of growing resistance against the recommended treatment drug combination.
The enabling environment for clinical trials
The protection of the health and rights of participants – healthy individuals and patients – should be the foremost concern of all involved in conducting, approving or funding clinical research. As a funder of clinical research EDCTP addresses this responsibility in various ways.
Of course, EDCTP-funded trials need to be conducted in accordance with national and international regulations and the rules for Good Clinical Practice (GCP) which are established by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), as well as good participatory practice.
Moreover, EDCTP invested and continues to invest in the environment which is needed to oversee clinical research taking place in sub-Saharan Africa:
- EDCTP supports the establishment and strengthening of committees for ethical review of clinical studies, national ethical review boards and regulatory bodies in sub-Saharan Africa through its ‘ethics’ grant scheme.
- EDCTP is a partner in the EU-funded TRUST project which aims to support adherence to high ethical standards in research globally and to counteract the application of double standards in research by co-developing with vulnerable populations, tools and mechanisms for the improvement of research governance structures.
- Equitable research partnerships are supported through investments in the development of clinical research capacity in sub-Saharan Africa research, either through investments in training integrated with clinical trials or through career development, clinical R&D, or senior fellowships.
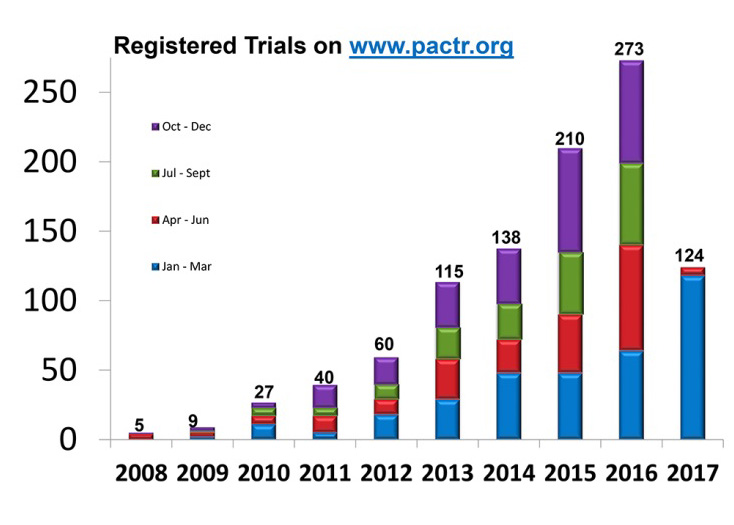
- Transparency was supported through main funding for the Pan-African Clinical Trials Registry (PACTR), the only WHO-endorsed primary registry in Africa.
- With other international funders, EDCTP contributes to further transparency and information sharing by providing its funding data (from 2012) to the World RePort, a web-based platform that maps biomedical research projects supported by major national and international funders such as the National Institutes of Health (USA), the Bill & Melinda Gates Foundation (USA), the European Commission, and Wellcome Trust (UK).
More information
- Signatories to the joint statement on 18 May 2017: Indian Council of Medical research, Research Council of Norway, UK medical research Council, Médecins sans frontiers, Epicentre, CEPI, Path, Institut Pasteur, Drugs for Neglected Diseases initiative (DNDi), Bill & Melinda Gates Foundation, and Wellcome Trust.
- Joint statement on public disclosure of results from clinical trials, International Clinical Trials Registry Platform (ICTRP).
