International Women’s Day 2023: Innovation and technology for gender equality
As we celebrate this year’s International Women’s Day, EDCTP wishes to express its commitment to the theme ‘DigitALL: Innovation and technology for gender equality’. Women are particularly disadvantaged and underrepresented in these areas. Therefore, EDCTP has been very intentional about increasing female active participation and growth in its funding programmes, which has resulted in a sharp increase throughout the programme’s lifespan.
According to the UN Women’s Gender Snapshot 2022 report, “women’s exclusion from the digital world has shaved $1 trillion from the gross domestic product of low- and middle-income countries in the last decade—a loss that will grow to $1.5 trillion by 2025 without action.” Women are notoriously underrepresented in science, and although numbers vary per country, it is estimated that only 18 – 31% of science researchers in sub-Saharan Africa are women. A Nature series on African women working in science notes:
“Women in Africa experience greater barriers to developing careers in science, technology, engineering and mathematics (STEM) than do women in high-income countries, with lack of funding a particular problem. Some challenges, however, will be familiar to women the world over. Many women need to take time out for pregnancy, maternity leave and breastfeeding, and women also tend to do a higher share of childcare and domestic duties.”
Increasing female participation
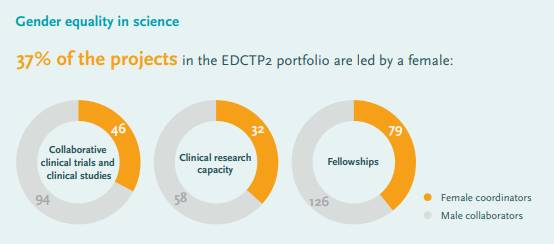
EDCTP has been very intentional about increasing female participation in its funding programmes. 37% of EDCTP2-supported projects are led by women, and 49% of the 692 African academic trainees (Bachelor’s, Master’s and PhD students) supported by EDCTP are female. In addition, 39% of EDCTP technical reviewers are female.
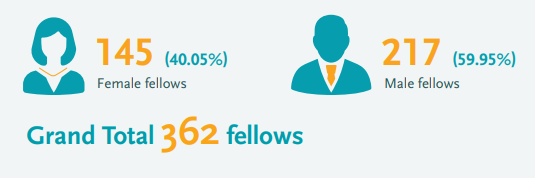
EDCTP’s role in promoting gender equity has grown over time. Of special note is EDCTP’s Fellowship programme, which saw a sharp increase in female participation from the first programme running from 2003-2015 (22% of EDCTP1 fellows were women) to the second programme running from 2014-2024 (40% of EDCTP2 fellows are female researchers):
Women in innovation and technology
Female leadership is reflected in numbers, but also in the variety of areas in which they are making a difference. The case studies below present just a small sample:
Women in epidemiology and biostatistics
For decades, Africa has faced challenges in responding to public health emergencies. Epidemiological data is often unavailable or severely limited, and there is a shortage of skilled personnel and systems to collect and analyse available data and translate them into policy and practice. To address this capacity gap, EDCTP and Africa Centres for Disease Control and Prevention (CDC) partnered in a €7.5 million initiative to boost epidemiological and biostatistical capacity on the African continent through Master’s degree programmes in epidemiology and biostatistics. Ten consortia were selected to provide this ‘EPI-Biostat’ training, comprising 38 African and 8 European institutions. Currently, they are training 151 MSc candidates in epidemiology and biostatistics: 63 females (42%) and 88 males (58%).
We asked one of the EPI-Biostat Fellows, Mrs Jeomba Mwakondja Alisa, how she thinks women can claim their role in science and technology.
Women in health systems management
Mrs. Delese Mimi Darko was awarded an EDCTP Ethics and Regulatory activities grant for the BERC-Africa project, building and enhancing regulatory capacity in Ghana. With over 30 years of experience, Mrs. Darko rose through the ranks at the Food and Drugs Authority (FDA) Ghana to become the first female Chief Executive Officer in 2017. Mrs Darko was instrumental in designating the FDA as a Regional Center of Regulatory Excellence, and the establishment and implementation of the Food Safety Policy and Food Emergency Response Plan in Ghana. Under her leadership, the FDA has introduced many innovations including the Progressive Licensing Scheme, in collaboration with the Ghana Enterprise Agency (GEA). She currently chairs the Steering Committee of the WHO African Vaccines Regulatory Forum (AVAREF) and serves on several international committees including Global Advisory Committee on Vaccine Safety (GACVS), the West African Medicines Regulatory Harmonization Steering Committee.
We asked Mrs Delese Mimi Darko how she navigated the path towards leadership of key national and international institutions.
Women in data
Professor Helen Ayles led the TREATS project, which investigated whether combined HIV/AIDS and tuberculosis (TB) interventions targeting entire populations have an impact on the burden of TB. Nested within the study, qualitative and economic data collection and evaluation of improved diagnostic tools for TB were included. Thus, data management and statistical analysis were key, allowing rigorous analysis and triangulation of the data, which can also be used in mathematical and economic modelling to predict the impact and cost-effectiveness of this approach.
The majority of the research team members from the TREATS consortium are women. We asked Professor Ayles about her experiences in working with this largely female team.