World AIDS Day 2017: EDCTP invests in clinical research to support health for all
Significant gains have been made in tackling the HIV epidemic: deaths from AIDS-related causes declined by 48% between 2005 and 2016, with the sharpest decline recorded in eastern and southern Africa. However, the decline in new infections is not rapid enough, and the United Nations’ Fast-Track Target of fewer than 500 000 new infections per year by 2020 may not be achieved (UNAIDS Data 2017).
“Research is key to ending the HIV epidemic. Biomedical research to develop and test new tools is vital now but so is implementation research to determine how best to deliver already-proven HIV prevention and treatment. People vulnerable to HIV exposure and the millions of people already living with HIV need diverse options for prevention and treatment. It is our joint responsibility to meet this global challenge and leave no one behind.”
Prof. Catherine Hankins, Chair of the EDCTP Scientific Advisory Committee
Health research supports the right to health for all
Research and development has a critical role in identifying, testing, and implementing effective interventions that support the right to health for all. EDCTP’s strategic research agenda addresses treatment, prevention, and product-focused implementation research with prioritisation of the following needs:
- Optimised treatment regimens and drug formulations for key groups such as children, pregnant women and adults with co-infections and co-morbidities;
- Increased access to proven interventions; and
- Novel HIV prevention strategies such as pre-exposure prophylaxis and vaccines.
“An urgent need remains for concerted efforts to develop and improve medical interventions for diagnosis, prevention and treatment for HIV and HIV-related co-infections, and to ensure that existing tools are optimally implemented to maximise their life-saving potential. EDCTP remains committed to building its R&D investment in this area and supporting international collaborations that address this need, especially in the world’s most vulnerable populations.”
Dr Christy Comeaux, EDCTP lead project officer for HIV
EDCTP-supported HIV clinical research impacts
EDCTP supported the REMSTART trial under EDCTP1. This trial provided data supporting the July 2017 WHO guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. REMSTART (Reduction of early mortality among HIV-infected subjects starting antiretroviral therapy: a randomised trial), which took place at sites in Zambia and Tanzania and was coordinated by Dr Saidi Egwaga, evaluated a health service strategy designed to reduce the high early mortality associated with antiretroviral therapy in Africa. A component of the strategy was an increased frequency of diagnostic testing for cryptococcal meningitis and TB. REMSTART demonstrated that all-cause mortality at 12 months was decreased in the intervention group when compared with the control group (in which participants were offered standard care). EDCTP continues to support investigation of this approach through our funding of the TRIP study (Translating Research Into Practice), led by Dr Sayoki Godfrey Mfinanga (National Institute for Medical Research, Tanzania). TRIP aims to determine the feasibility, cost-effectiveness, and impact of scaling up the REMSTART intervention in 12 urban and 6 rural settings in Tanzania.
Many people living with HIV are also affected by diseases such as TB, a co-infection which has been—and continues to be—well researched. However, not all HIV-related co-infections have received sufficient attention. Despite significant strides in HIV management, opportunistic infections are still a frequent cause of mortality in immunosuppressed people living with HIV. Due in part to the highly immunocompromised status of these patients, co-infections can result in unique diagnostic and treatment challenges. EDCTP has responded to this challenge through support for research on HIV-associated co-infections—especially cryptococcal infections—and has consulted widely with stakeholders in the field to guide our investments in this area. Currently, EDCTP-supported projects on HIV-associated co-infections include the DREAMM and the AMBITION projects.
The DREAMM project, led by Dr Angela Loyse (St. George’s University of London, UK), is a multi-centre study that aims to evaluate a semi-quantitative cryptococcal antigen lateral flow assay (CrAG LFA) called Biosynex crypto PS, developed by the Pasteur Institute (France). This test will enable the identification of HIV patients with high CrAg cryptococcal meningitis titres, who may benefit from more aggressive or prolonged antifungal therapy. The CrAg LFA test is embedded within an algorithm that aims to reduce time to diagnosis and time to initiation of effective treatment. DREAMM is taking place at sites in Cameroon, Malawi, and Tanzania, and includes partners from the National Institute for Medical Research – Tanzania, Lilongwe Medical Relief Trust Fund, the Central Hospital of Yaoundé (Cameroon), Institute Pasteur, and the Liverpool School of Tropical Medicine.
The AMBITION project, led by Dr Joseph Jarvis (London School of Hygiene & Tropical Medicine, UK) is funded by EDCTP and from the UK by MRC, DFID, and Wellcome Trust. AMBITION is a multi-centre phase-III trial to determine whether short-course high-dose liposomal amphotericin (L-AmB, Ambisome) is as effective as 14-day amphotericin B-based therapy in averting all-cause mortality in HIV-associated cryptococcal meningitis. Eight hundred and fifty patients will be recruited at 6 African partner-sites, making this the largest HIV-associated cryptococcal meningitis trial ever conducted. A novel short-course highly effective and safer L-AmB treatment regimen for cryptococcal meningitis would transform the management of late-stage HIV and markedly improve outcomes in HIV programmes in Africa. Project partners include the Liverpool School of Tropical Medicine, the University of Liverpool, St. George’s University of London, Institut Pasteur, Infectious Diseases Institute Limited, the Botswana-Harvard AIDS Institute Partnership, University of Cape Town, University of Zimbabwe, and Lilongwe Medical Relief Trust Fund.

Summary of investments
During the first EDCTP programme (2003–2015), EDCTP allocated €61.41 million to 56 HIV-related grants, including 30 clinical trials on HIV and 9 on HIV/tuberculosis (TB) co-infection.
By the end of 2017, the second EDCTP programme (2014-2024) had already invested nearly €40 million in 15 grants on HIV research. A further €40 million has been allocated to 15 grants selected for funding (contracts not yet concluded).
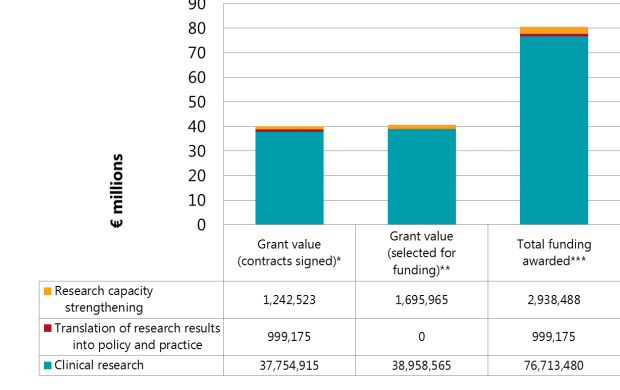
Notes to the graph
* Contracts signed – grant agreements have been concluded and funding disbursed.
** Selected for funding – grant agreements have not been concluded but projects have been selected for funding.
*** Total funding awarded – the sum of all funding already disbursed and potentially to be disbursed to grants selected for funding.
More on the EDCTP portfolio
The EDCTP2 programme is supported under the Horizon 2020, the European Union’s Framework Programme for Research and Innovation.
EDCTP’s public Project Portal provides an up-to-date view on the projects supported as our portfolio grows, and gives details of each project’s aims and objectives. Search “HIV” or “Co-infections” in the “classification” box to see current HIV projects.
The portfolio of projects supported under the first EDCTP programme can be viewed here.
The TRIP, DREAMM and AMBITION-cm projects were highlighted at the EDCTP-ANRS symposium at the 9th International AIDS Society Conference on HIV Science 2017 (see programme brochure). See the October 2017 issue of the EDCTP Newsletter for a report on the symposium.
