World AIDS Day 2020
On World AIDS Day 2020, the significant progress achieved over the last two decades is justly celebrated. However, the HIV-epidemic is still far from being under control. Major challenges remain to bring down the rate of new infections and reach all those that are infected with adequate interventions. The most recent report by UNAIDS – Seizing the moment: Tackling entrenched inequalities to end epidemics, published on 26 November 2020 – makes clear that HIV remains a major global public health issue. The world did not achieve the intermediate objectives for 2020 and is not on track to end the HIV epidemic by 2030.
Moreover, due to the COVID-19 pandemic, HIV prevention, testing, treatment and care services are being disrupted particularly in countries with fragile health systems. Many vulnerable populations will be left at greater risk of HIV infection and AIDS-related deaths in case of a breakdown in essential HIV services.
“During this time of crisis, a balanced and holistic approach is key. We need to maintain investments in research and development, as well as in the most critical prevention activities and health-care services for HIV and other related diseases, to substantially reduce the overall impact of the COVID-19 pandemic.”
Dr Michael Makanga, EDCTP Executive Director
EDCTP investments in HIV research
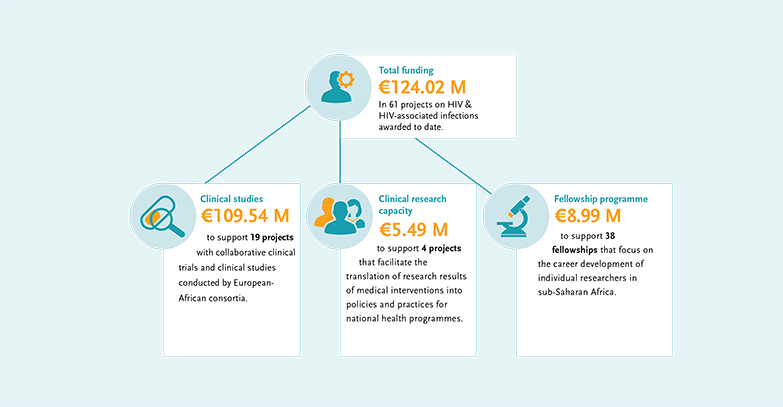
The 61 EDCTP-funded studies on HIV and HIV-associated infections represent a total investment of
€124.02 million. Of this amount, €109.54 million supports nineteen large projects with collaborative clinical studies conducted by European-African consortia. Additionally, four projects facilitate the translation of research results into HIV policies and practices for national health programmes receiving a total of €5.49 million. The 38 fellowships for individual HIV-focused researchers in sub-Saharan Africa are supported with a total of €8.99 million.
In its detailed World AIDS Day message, the World Health Organization pays a “tribute to all those working to provide HIV services and calls on global leaders and citizens to rally for ‘global solidarity’ to maintain essential HIV services during COVID 19 and beyond”. Furthermore, WHO calls for innovation and integration of HIV services and prioritising vulnerable groups, including pregnant women, adolescents, and youth. EDCTP is confident and proud that its HIV portfolio is aligned with this global agenda. This message highlights investments in innovative prevention and the needs of key vulnerable populations, especially adolescents.
Innovation and prevention
Ongoing high rates of HIV-infection in young women in sub-Saharan Africa remain an obstacle to reaching the UNAIDS goal of ending the AIDS epidemic by 2030. New technologies for HIV prevention in women are therefore identified as a high priority in HIV research. While daily oral pre-exposure prophylaxis (PrEP) has been proven effective, adherence challenges remain. The development of safe and effective long-acting agents would increase HIV prevention options.
In perhaps the best HIV news this year, the HTPN 084 study recently found that a regimen containing long-acting cabotegravir injected once every eight weeks was superior to daily oral tenofovir-emtricitabine at preventing HIV in cisgender women. The study has been conducted by the HIV Prevention Trials Network, a worldwide collaborative clinical trials network funded by several National Institutes of Health (United States).
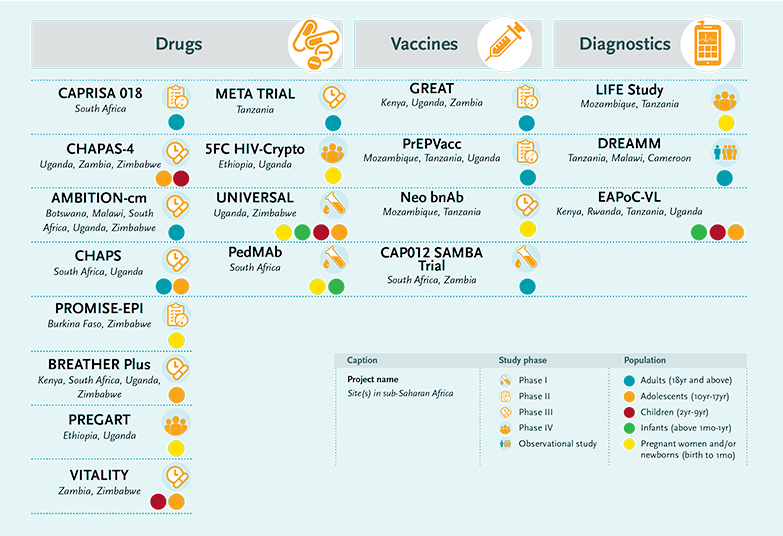
Sustained-release sub-dermal implants could be an alternative to long-acting injectables. The EDCTP-funded CAPRISA 018 trial is investigating the safety and acceptability of a sustained release tenofovir-alafenamide sub-dermal implant for HIV prevention in women that would last six months. As sub-dermal implants are already widely available through family planning service in several sub-Saharan African countries, implementation could be relatively straight forward.
A different route to prevention is by passive immunisation with monoclonal antibodies, as investigated in another EDCTP-funded CAPRISA study CAP012 SAMBA. In this trial, safety and acceptability are evaluated for three leading broadly neutralising antibodies (bNAbs). The most promising combination(s) of bNAbs will then proceed to phase II to assess extended safety and obtain an efficacy estimate of preventing HIV infection in young women. It offers the prospect of HIV protection delivered through injections every four to six months.
Innovative vaccine research is being conducted in two important phase II studies. The PrEPVacc trial is combining drugs and vaccines. It will assess the impact of two experimental HIV vaccine regimens, already tested and shown to be safe in people, when used alongside antiretroviral-based PrEP. The trial will be carried out in high-risk populations in four sub-Saharan African countries. The GREAT project will test the safety of the candidate vaccine tHIVconsvX, as well as its ability to generate HIV-specific immune responses. The project team will work with a range of marginalised communities, including fishing communities around Lake Victoria, male and female sex workers, and men who have sex with men. Together, these studies represent a combined project value of €39 million to which EDCTP contributed more than €22 million.
Innovative interventions are not adopted as a matter of course. To address implementation challenges, the EDCTP-funded UPTAKE project investigates ‘Universally accessible HIV prevention technologies for African girls and young women through knowledge applied from behavioural economics’. The project focuses on prevention including injectable and implantable long-acting PrEP; the coordinator is Dr Anatoli Kamali at IAVI. The project aims to strengthen the connections between new product developers and vulnerable user communities and brings together a collaboration of academic, public health, and product development organizations.
Adolescents: prevention and treatment
The CHAPS project is evaluating a range of strategies suitable for adolescents. These include a modified version of PrEP. Although daily PrEP reduces the risk of HIV infection, adherence is a challenge for adolescents. The CHAPS project evaluates the impact of an alternative, less toxic drug combination (tenofovir alafenamide and emtricitabine) as well as consulting with adolescents to determine their attitude to and acceptance of ‘on-demand’ PrEP – use of PrEP just around the time of sexual activity. Laboratory-based studies are being used to optimise dose levels and dosing schedules for the novel antiretroviral drug combination.
The BREATHER Plus trial will compare two new approaches – weekends off and a monthly injectable – with standard treatment in adolescents in four African countries. The oral regimens will be based on dolutegravir, an increasingly used antiretroviral. Importantly, the trial will take place in settings where viral load is monitored annually, as recommended by WHO, so any resurgence of HIV can be detected. The trial may improve the treatment and quality of life of more than 2 million adolescents living with HIV – most of whom are in sub-Saharan Africa.
The VITALITY project is the first large-scale investigation in sub-Saharan Africa to determine whether supplementation with vitamin D3 and calcium carbonate can improve musculoskeletal health in young people aged 8–16 years with HIV infection. In addition, vitamin D supplementation may have beneficial effects on the immune system and on the development of healthy microbial communities in the gut (the microbiome).
Conducted in Zambia and Zimbabwe, the project will generate data on a simple, safe and affordable intervention that could have significant benefits for people living with HIV. Vitamin D3 has the potential to provide short- and long-term social, economic and health benefits.
EDCTP Fellow Dr Grace McHugh aims to investigate innovative age-appropriate strategies and youth-friendly approaches to explore the feasibility and acceptability of HIV self-testing (the FAST study). She is investigating the effectiveness of two different community-based strategies for delivering HIV self-testing to adolescents and young people at educational institutions and within communities, using peer distribution.
HIV and COVID-19
Taking advantage of the infrastructure provided by the EDCTP-funded TREATS project, the TREATS-COVID project is gaining a deeper understanding of the spread of COVID-19 in an urban population of Zambia. The TREATS project is working closely with a community of around 28,000 that has been involved in research studies for many years.
All households will be visited, and people aged 15 years and above will be screened for symptoms of COVID-19. Cases and their household contacts will be followed up. A second study will test 4000 people for past infection, as well as for HIV and TB infection.
The project will evaluate a range of potential community-based point-of-care tests to detect SARS-CoV-2 and antibodies to the virus. These will include a novel computer-aided diagnostic tool for interpretation of chest X-rays that has been developed by one of the project partners, initially for TB but since adapted for COVID-19.
The HALT-COVID project aims to determine the impact of HIV and TB infections on COVID-19 symptoms, outcomes and transmission, and how COVID-19 affects morbidity and mortality in populations badly affected by HIV and TB.
The project is based on two study populations, in rural and urban areas, that are already participating in a surveillance project. Through the use of existing mobile clinics, 5,000 people with COVID-19-like symptoms will be offered molecular testing. In addition, healthcare workers at two research institutes will be offered routine weekly screening.
An important aspect of the project is to evaluate molecular and antibody-based tests in the South African setting. Focusing on the most practical tests, the project will assess their performance against gold standard comparators.
The TraCE project aims to follow 120 people with confirmed COVID-19, as well as their household contacts, in a resource-limited, densely populated community in Cape Town, South Africa. The project uses well-established mobile screening units, which were set up to provide HIV counselling and testing services. These, along with public sector clinics, will be used to identify cases.
A total of 120 households will be recruited and followed for one month, with half receiving an intensive infection mitigation intervention administered by lay health workers and the other half being given standard advice on infection prevention. The intervention includes guidance on infection control, basic supplies (such as masks and hand sanitiser), and regular telephone calls and text messages.
The periCOVID-Africa project aims to generate key data on COVID-19 in pregnant women and newborn babies, taking advantage of two established cohorts in sub-Saharan Africa. The first cohort set up through the EDCTP-funded PREPARE project covers 70,000 pregnant women and their infants in Kampala, Uganda.
The PREPARE project is following up women from the early stages of pregnancy and infants up to the age of three months to gain a clearer picture of the burden of group B streptococcus infections.
The second cohort is drawn from a multicentre study of 10,000 pregnant women in The Gambia, Kenya and Mozambique organised by the PRECISE Network, with support from UK Research and Innovation and Wellcome Trust. This project is exploring the social, clinical and biological factors associated with a range of placental disorders, such as low birth weight and stillbirth.
Subsets of participants in these studies will be screened for COVID-19 symptoms and invited to join the periCOVID-Africa project if they test positive. The project will provide a picture of COVID-19 infections in pregnant women in five different settings across sub-Saharan Africa, helping to establish the maternal, foetal and neonatal burden of disease in representative sub-Saharan Africa populations.
All projects have been funded under EDCTP’s 2020 COVID-19 Emergency call for proposals. Details on the coordinators and funding can be found on the EDCTP website page Mobilisation of funding for COVID-19 research in sub-Saharan Africa.
More information
- UNAIDS – 2020 Global AIDS Update – Seizing the moment: tackling entrenched inequalities to end epidemics
- WHO – World AIDS Day 2020
- EDCTP portfolio: HIV and HIV-associated infections (online)
