World Malaria Day 2014: ‘Invest in the Future. Defeat Malaria’
On the occasion of World Malaria Day 2014, the World Health Organization calls on all to continue investing in the future and defeat malaria which still kills an estimated 627 000 people every year, mainly children under 5 years of age in sub-Saharan Africa. Global efforts to control and eliminate malaria have saved an estimated 3.3 million lives, reducing malaria mortality rates by 42% globally and 49% in Africa. Increased political commitment and funding have helped to reduce malaria incidence by 25% globally, and 31% in Africa. However, there is an urgent need to continued investments in malaria control and research in order to secure these gains and make further progress. EDCTP fully supports these goals.
The second EDCTP programme (2014-2024)
The second EDCTP programme (EDCTP2) is expected to commence in the second half of 2014. On 15 April, the European Parliament approved the participation of the European Union in EDCTP2. On 6 May, the Council is anticipated to approve the proposal as well. With a budget of € 683 million from the European Union to be matched by the participating Member States, the second programme will support all stages of clinical trials, from phase I to IV, on HIV/AIDS, tuberculosis, malaria and neglected infectious diseases.
The strategy for the second EDCTP programme as regards malaria will prioritise investments in research aligned with the threefold Global Malaria Action Plan. This plan focusses on control, elimination and research. In 2013, EDCTP organised a Stakeholder meeting on malaria in Vienna, Austria on 19-20 September 2013. It was co-hosted by the Austrian Ministry of Science and Research and the Medical University of Vienna and attended by 63 invited participants, including researchers from academia, representatives of product development partnerships and the pharmaceutical industry, policy makers, funding agencies and other like-minded organisations.
Overview of EDCTP investments in malaria research
The EDCTP malaria portfolio includes 12 vaccine trials that comprise phase I-II trials of several promising candidates, and 22 treatment trials of which include 12 phase IV (post registration) trials, mainly on label expansion, including studies on special or vulnerable groups such as pregnant mothers, very young or malnourished children and people living with HIV. Moreover, the approach adopted in these projects reflects the EDCTP strategy of integrating high quality research with investment in clinical capacity development and expansion of research networks in sub-Saharan Africa. As of 31 December 2013, the total funding for the 41 malaria research projects supported by EDCTP amounted to € 51 million.
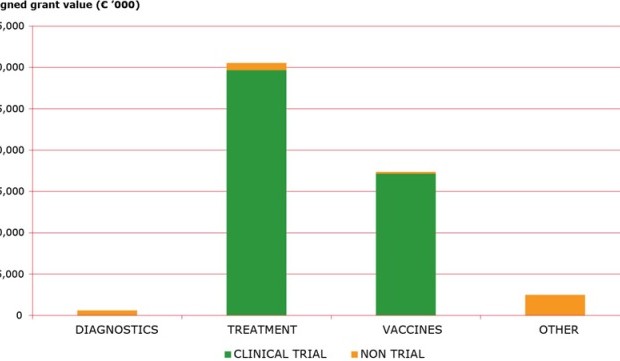
As resistance of malaria parasites to conventional antimalarial drugs is emerging, it is important to support development of new drugs and combination regimens. EDCTP provided € 19.53 million to support 19 projects on malaria treatment, which include studies of artemisinin-based combination therapies (ACTs) and non-ACTs. The studies aimed to establish therapies that are safe and highly effective in real life situations.
Moreover, current studies aim to address the needs of patient groups that need special attention such as infants, malnourished children, HIV/AIDS co-infected individuals and pregnant women. EDCTP funds three studies with a grant value of € 11 million focusing on malaria in this population which have been implemented by the Malaria in Pregnancy Consortium.
An effective and highly efficacious malaria vaccine is required to help reduce the burden of malaria in endemic countries. Since 2003, EDCTP has spent € 17.35 million to support six projects that include clinical trials of vaccines and projects that have a focus on immunological responses and capacity building for vaccine trials. Among these projects are the studies of the Malaria Vectored Vaccine Consortium (MVVC).
WANECAM: integrated approach to malaria treatment in West Africa
The West African Network for Clinical Trials of Antimalarial Drugs (WANECAM) includes four countries: Burkina Faso, The Gambia, Guinea and Mali and is funded by EDCTP with additional support from the Medicines for Malaria Venture (MMV), and several pharmaceutical and product development companies. The project is coordinated by Prof. Abdoulaye Djimdé.

The overall objective of the project is the development of a sub-regional network equipped with state of the art clinical trial sites, laboratories, research teams and well characterized populations ready to undertake Phase I–IV development of new drugs. The goals of malaria control may never be achieved without strong involvement of African scientists who are directly affected by this terrible disease in their daily lives.
The primary objective of the clinical study is to compare the incidence rate of uncomplicated malaria episodes in children and adults treated with repeated ACTs over a period of 2 years. In this 3-arm study pyronaridine-artesunate (PA) and dihydroartemisinin-piperaquine (DHA-PQP) will be compared to either artesunate-amodiaquine (ASAQ) or artemether-lumefantrine (AL) (depending on the site location). PA and DHA-PQP will not be formally compared.

Clinical trials are well underway in all three countries with seven study sites actively recruiting patients. Major infrastructure upgrades were conducted in all study sites. New state of the art laboratory equipment was provided to each of the seven trial sites. Short term training in various aspects of the study including ethics, good clinical and laboratory practices, molecular biology, data management, financial management were accomplished. The Guinea observational study was completed and data cleaning and analysis is underway. The project is expected to be completed in September 2014.
More information
- WHO World Malaria Day 2014 message
- WHO World Malaria Report 2013
- Roll Back Malaria: Global Malaria Action Plan for a malaria-free world
- EDCTP Stakeholder meeting on malaria report 2013
- Website of the Malaria in Pregnancy Consortium
- Website of WANECAM
