World Malaria Day 2015: funding research to close the gaps
According to the WHO World Malaria Report 2014, 198 million cases of malaria occurred globally in 2013 and the disease led to 584,000 deaths. The burden is heaviest in sub-Saharan Africa, where an estimated 90% of all malaria deaths occur. Children aged less than five years account for 78% of all malaria deaths.
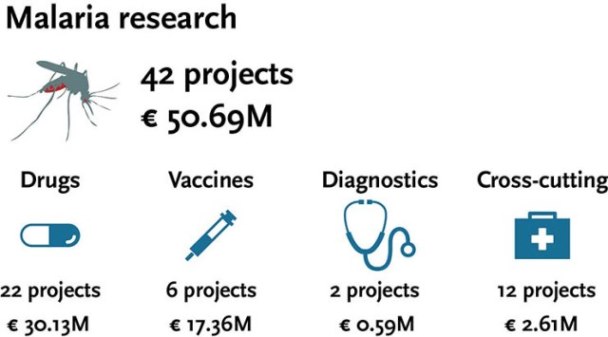
In its first programme, EDCTP funding strategy focused on clinical studies of high relevance to sub-Saharan Africa, in particular, research on malaria treatment in high-risk populations including children and pregnant mothers. Since 2003, malaria research has received €50.69 million of funding (23.9% of total EDCTP grant funding) through 42 grants.
EDCTP portfolio of malaria research
Antimalarial drug trials total up to 22, including prevention/chemoprophylaxis and treatment trials. Some trials are phase IV post-marketing studies evaluating the use of antimalarials in real-life settings in low-income countries.
Importantly, many other studies are phase IIIb clinical trials focusing on special populations such as pregnant women, children and HIV-infected individuals.
Trials evaluating optimisation of antimalarials in special populations for potential label extensions are very labour-intensive requiring extensive pharmacological analyses and a highly cautious approach in order to ensure patient safety. These studies are essential to produce evidence that will inform and guide future malaria prevention and treatment policies.
EDCTP is also active in the field of malaria vaccines with 12 clinical trials in the portfolio of the first programme. As a member of the Malaria Vaccine Funders Group (which was involved in the development of the Malaria Vaccine Technology Roadmap published in November 2013), EDCTP provides regular updates to the WHO Rainbow Table of malaria vaccines which are under development.
Malaria research funding under second EDCTP programme
The second EDCTP programme (EDCTP2) will continue to fund clinical research on malaria as it remains a major poverty-related disease in sub-Saharan Africa. However, the nature of the EDCTP calls for proposals has changed as compared to the first programme. Previously, EDCTP launched mainly disease specific calls.
Under EDCTP2, calls for proposals will be broader and applicable to all diseases within the scope of EDCTP, namely HIV/AIDS, tuberculosis, malaria and other poverty-related and neglected infectious diseases in sub-Saharan Africa. This approach creates more frequent funding opportunities for clinical malaria research.
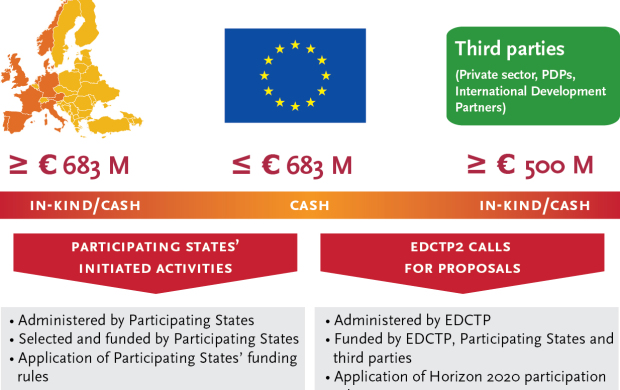
The European Union will contribute up to €683 million to the EDCTP2 programme, matching the €683 million commitment of the European countries participating in EDCTP2. Additionally, one of the EDCTP2 objectives is to leverage €500 million from third parties.
EDCTP publishes annual workplans which contain details on the activities to be funded including the calls for proposals.
Funding schemes
Three distinct types of funding schemes or ‘actions’ are supported under the EDCTP programme:
• Research & Innovation Actions (RIA) supports multicentre clinical trials that are conducted by research teams, with integrated capacity development and networking elements
• Coordination & Support Activities (CSAs) consists of capacity support activities that strengthen the enabling environment for conducting clinical trials and clinical research
• Training & Mobility Activities (TMAs) focus on the career development of individual researchers or clinical staff.
More information
- WHO World Malaria Day 2015 message: call to close gaps in prevention and treatment to defeat malaria
- WHO Africa Region: message of Regional Director Dr Matshidiso Moeti
- WHO World Malaria Report 2014
- Funding schemes on the EDCTP website
- EDCTP Work plan 2014 (PDF download)
