World Malaria Day 2020
On World Malaria Day 2020, the fight against malaria is facing stalled progress and a disruptive pandemic while the road to malaria eradication is still long and complex. This World Malaria Day is a call to keep malaria on the political agenda and for everyone involved to take ownership: “Zero malaria starts with me”.
In its World malaria report 2019, the World Health Organization (WHO) concludes that progress in malaria control has almost come to a halt. Over the period 2014-2018, the number of new infections did not decline and as many people died from malaria in 2018 as in the year before. The progress made earlier is currently threatened by the disruption caused by the COVID-19 pandemic. WHO urges countries to ensure the continuity of malaria services and to adapt malaria interventions to the pandemic response. Ahead of World Malaria Day 2020, WHO released an important report on reinforcing the global malaria eradication strategy to get back on target. Eradication of malaria needs to stay on the global agenda.
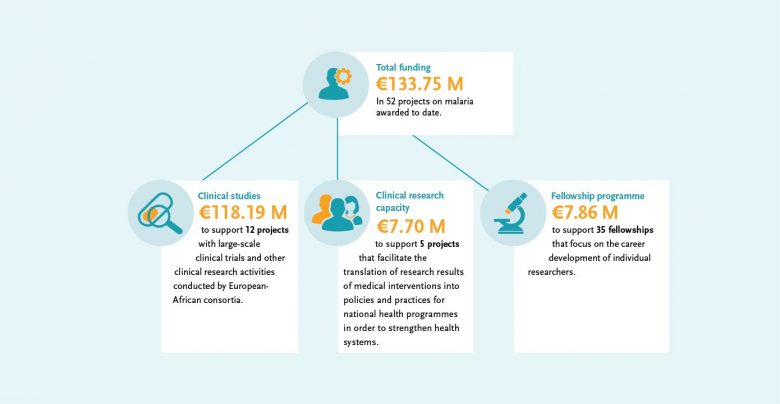
“EDCTP fully supports and embraces the WHO vision of a world free of malaria. To achieve this long-term and multifaceted objective, research and development for new and improved transformative tools aimed for use in an integrated and multidisciplinary manner is essential. EDCTP has already supported this R&D agenda with investments in clinical malaria research of almost €134 million.”
Dr Michael Makanga, EDCTP Executive Director
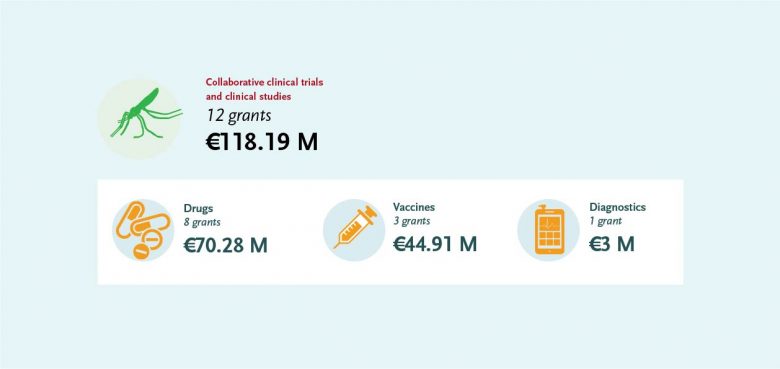
The EDCTP malaria portfolio comprises 52 projects, from large-scale international collaborative clinical research projects implemented by African-European research consortia, through to 35 projects conducted by EDCTP Fellows, who are leading the malaria research agenda in Africa. The EDCTP portfolio includes several large-scale projects testing some of the most advanced new vaccine and drug candidates that could contribute significantly to the malaria eradication agenda.
Highlights of clinical malaria research
DIAGNOSTICS
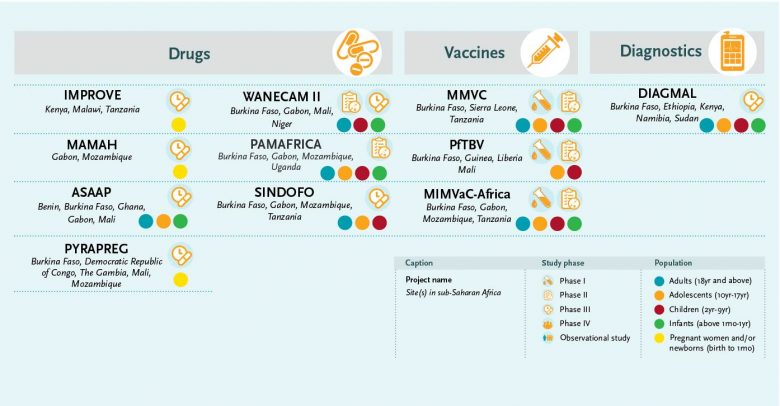
Malaria diagnostics research is supported with a large grant of almost €3 million to the malaria diagnostics consortium DIAGMAL. The project is led by Dr Henk Schallig (Amsterdam University Medical Centre, the Netherlands) and aims to assess the diagnostic accuracy of a small molecular diagnostic test – the ‘mini-db-PCR-NALFIA’ – in five different endemic settings. The platform, controlled via a mobile telephone, does not require DNA extraction, has a simple read-out system, can operate on a battery and may be used as a point-of-care tool. The test has passed the first two phases of diagnostic evaluation. Its diagnostic accuracy will be assessed through a phase III diagnostic trial.
TREATMENT
The IMPROVE study is a pivotal study for preventive treatment of malaria in pregnancy. After a decade of trials to find new prevention strategies, dihydroartemisinin-piperaquine (DP) has been shortlisted as the only potential alternative to sulphadoxine-pyrimethamine (SP) for Intermittent preventive treatment in pregnancy (IPTp), but evidence of its benefits on infant outcomes is needed.
The aim of the study is to evaluate the safety, tolerance and beneficial effects of IPTp with DP (alone or combined with azithromycin). Acceptability, feasibility and economic studies will explore the potential for integration and scalability within the national malaria control programmes. The results may provide WHO with definitive evidence to determine whether the new treatment approach is a viable alternative to IPTp with SP, especially in areas of the world with moderate to high malaria transmission and high parasite resistance to SP.

The project is led by Professor Feiko ter Kuile (Liverpool School of Tropical Medicine, United Kingdom). The multi-centre clinical trial is conducted in 11 hospitals in Kenya, Tanzania and Malawi. Approximately 4,680 pregnant women have been enrolled with most fieldwork completed just before the COVID-19 public health crisis started.
IMPROVE is a collaboration of partners from Denmark, Finland, Kenya, Malawi, Norway, Tanzania and the United Kingdom. EDCTP awarded a €7.4 million grant to the project, while additional funding of £2.7 million from the Joint Global Health Trials (JGHT) made it possible to include a trial with HIV-infected pregnant women alongside the main study with HIV-uninfected participants. JGHT is a partnership of the UK Department for International Development, the UK medical Research Council, the National Institute for Health Research and the Wellcome Trust.
VACCINES
EDCTP funds three large portfolio projects on malaria vaccine development with a total value of €45 million. The projects focus on a number of candidate vaccines against various stages of the malaria parasite.
MMVC, the Multi-Stage Malaria Vaccine Consortium, is led by Professor Adrian Hill from Oxford University, United Kingdom, and aims to develop the first multi-stage vaccine for malaria designed to target all four stages of the Plasmodium falciparum parasite’s life-cycle. This highly ambitious project encompasses a series of tightly coordinated lead-in trials building towards a phase IIb efficacy trial. Since 2018, a number of trials have been initiated in Kenya, Tanzania and Burkina Faso with promising preliminary results obtained for the pre-erythrocytic candidate R21/Matrix-M.
The MIMVaC-Africa consortium started in February 2020 with a highly ambitious programme to conduct clinical trials on a portfolio of five malaria vaccine candidates: three pre-erythrocytic vaccine candidates (R21/Matrix-M, the chemically attenuated whole sporozoite vaccine and the ME-TRAP viral vectors) and two blood-stage candidates (PfRH5/Matrix-M and the NPC-SE36/CpG).
The project is led by Dr Sodiomon Bienvenu Sirima of the Groupe de Recherche Action en Santé (GRAS), Burkina Faso. The consortium will leverage its recently developed capacity to work with the controlled human malaria infection (CHMI) challenge model. The project will rapidly test and compare the vaccine candidates in Europe and Africa in order to select the most promising for the field efficacy trials in Africa. The MIMVaC-Africa programme will also strengthen the research capacity of the African clinical research sites.
The PfTBV consortium is coordinated by Dr Issaka Sagara of the University of Sciences, Techniques and Technologies in Bamako, Mali, with research sites in Burkina Faso, Guinea, Liberia and Mali. The consortium aims to evaluate a portfolio of three innovative candidate malaria vaccines that aim to block transmission of the parasite with antigens (Pfs48/45, Pfs230 and a Pfs230-Pfs48/45 chimeric antigen). Recently, its first year of activities was completed; a phase II community trial is ongoing to determine if the experimental malaria transmission blocking vaccine (Pfs230D1M-EPA/AS01) is safe and can provide a protective immune response in Malian children and adults.
FELLOWSHIPS
Several important studies are conducted by recipients of EDCTP fellowship grants. The EDCTP fellowship scheme aims to support the development of scientific leadership in sub-Saharan Africa and to provide up-and-coming researchers with the opportunity to develop their clinical research skills.
Professor Faith Osier has created an international network to map the malaria parasite diversity across Africa, a key step in the development of more effective malaria vaccines. More in the EDCTP online portfolio.
Dr Dominic Mosha evaluates the safety of a single low-dose primaquine co-administered with ACT in routine healthcare practices. The aim is to study the implementation of a WHO-recommended treatment regime. More in the EDCTP online portfolio.
Dr Laurent Dembele aims to establish a screening model for malaria liver-stage infection by Plasmodium vivax and Plasmodium ovale using field-isolated sporozoites from infected mosquitoes. More in the EDCTP online portfolio.
Projects of EDCTP member countries
The EDCTP2 programme includes projects that are initiated and funded by EDCTP member countries independently. These so-called Participating States’ Initiated Activities (PSIAs) are considered a contribution to the programme if they are within the scope of the EDCTP programme and contribute to its objectives.
The United Kingdom funded through the JGHT scheme the first randomised controlled trial to provide evidence that PBO-treated long-lasting insecticidal nets (synergist piperonyl butoxide) were more effective than standard pyrethroid long-lasting insecticidal nets against malaria infection and transmission. The trial results prompted WHO to revise its policy on long-lasting insecticidal nets in September 2017, give interim endorsement to pyrethroid-PBO nets as a new WHO class of vector control product, and recommend that PBO nets be deployed for prevention of malaria where vectors are resistant to pyrethroids if vector control coverage is not compromised. (PSIA-2014-751)
Sweden funded the ‘Sweden-Kenya science venture for malaria eradication in tropical Africa’ programme. This was a collaboration between Kenya, Japan and Sweden to establish a national malaria elimination strategy in Kenya. It addressed challenges related to mass drug administration, such as long-term surveillance of drug safety and efficacy, drug resistance, prevention of parasite importation and management of resurgence. Local health facilities have been strengthened to support malaria elimination programmes. (PSIA-2014-619)
Spain supported research activities in Mozambique related to the RTS,S vaccine, the world’s first licensed malaria vaccine. One of the studies funded showed that RTS,S continues to protect young children and newborn babies against clinical malaria for up to 18 months after vaccination. Moreover, in the area of malaria treatment, studies on the antimalarials artemether-lumefantrine and artesunate-amodiaquine found very high efficacy for both treatments, confirming their position as the first- and second-line treatments for malaria, respectively. (PSIA-2014-718)
More information
- WHO – World Malaria Day 2020
- WHO – World Malaria Report 2019
- WHO – Tailoring malaria interventions in the COVID-19 response
- WHO – Strategic Advisory Group on Malaria Eradication Malaria eradication: benefits, future scenarios & feasibility
- EDCTP Fellowships. See the EDCTP online portfolio: Fellowship programme and the EDCTP Alumni Network platform
- Sweden – PSIA-2014-619 | Sweden-Kenya science venture for malaria eradication in tropical Africa | Project online fact sheet
- United Kingdom – PSIA-2014-751: Evaluation of a Novel Long Lasting Insecticidal Net and Indoor Residual Spray Product | Clinical trial registration | Publication
- IMPROVE – Project website
- MMVC – Project pages on Jenner Institute website
- PfTBV – Project website
