World Malaria Day 2023: time to deliver zero malaria
Today, partners all around the world are reaffirming their commitment to eradicate malaria, as expressed in the theme of this year’s World Malaria Day: “Time to deliver zero malaria: invest, innovate, implement”. EDCTP joins WHO in its focus on the third “i” – implement – and more specifically on advancing tools and strategies to reduce the burden of malaria among vulnerable populations that are often excluded from clinical studies but that have major unmet medical needs. We do so by funding the development of innovative products – diagnostics, drugs and vaccines – and the implementation of interventions and activities to strengthen health systems.
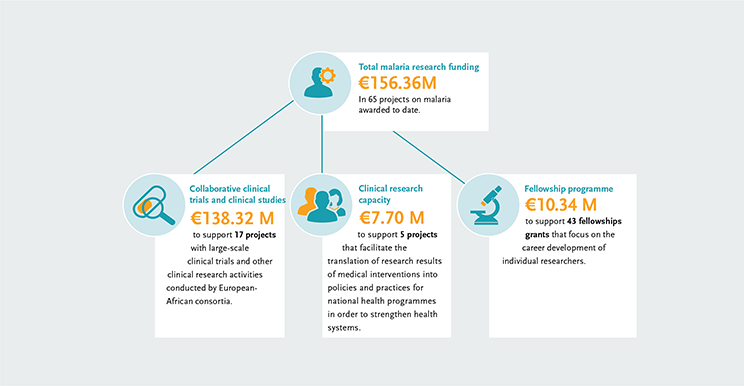
The EDCTP malaria portfolio comprises 65 projects, from large-scale international collaborative clinical research projects implemented by African-European research consortia, to projects conducted by EDCTP Fellows who are leading the malaria research agenda in Africa. Our portfolio includes projects testing some of the most advanced new malaria vaccines and drug candidates that could contribute significantly to the malaria eradication agenda. In addition, we support several large-scale multinational implementation and operational research interventions.
Vulnerable populations
Malaria disproportionately affects the most marginalised populations in society.
In recognition of this fact, EDCTP has put an emphasis on funding research to advance the health of these vulnerable populations. The EDCTP programme encompasses populations often excluded from clinical studies but with major unmet medical needs – including pregnant women, newborns, children, other vulnerable populations, and people with co-infections and comorbidities.
According to the World malaria report published by WHO on 8 December 2022, the African region carries the heaviest burden of the disease – accounting, in 2021, for an estimated 95% of all malaria cases (234 million) and 96% of all deaths (593 000). Nearly 80% of malaria deaths in the African region were among children under the age of 5. We invite you to explore the following case studies of projects that focus specifically on implementing tools and strategies to reduce the burden of malaria, in particular, among pregnant women, infants and children.
Booster dose of R21/Matrix-M malaria vaccine candidate highly efficacious (MMVC study)
The Multi-Stage Malaria Vaccine Consortium (MMVC) have reported in The Lancet Infectious Diseases that a booster dose of the R21/Matrix-M malaria vaccine candidate at one year following the primary three-dose regimen maintains high efficacy. This follows the results of the phase II trial of R21 in Burkina Faso which showed vaccine efficacy of 77% in children aged 5–17 months over a year with a higher-dose of Matrix-M adjuvant, with no significant safety issues. MMVC is developing the first multi-stage vaccine for malaria, designed to target different stages of the Plasmodium falciparum parasite.
As well as the impressive efficacy of R21/Matrix-M, the first vaccine to achieve the WHO’s target of 75% efficacy, R21/Matrix-M may have an important advantage over RTS,S/AS01 in that it can be used at significantly lower doses, which should boost vaccine supply.
New antimalarial better tolerated, safer and more effective (IMPROVE study)
Malaria in pregnancy can have devasting consequences for the mother and foetus, resulting in severe anaemia in the mother, maternal death, or the mother losing the pregnancy or the baby being born too early or too small. These premature and low birth weight babies have a four times higher risk of dying during their first year of life. WHO recommends the use of intermittent preventive therapy during pregnancy (IPTp) as prophylactic preventive intervention. IPTp is used in 35 countries in sub-Saharan Africa but the malaria parasite has become increasingly resistant to the only drug currently recommended by the WHO for IPTp: sulfadoxine-pyrimethamine (SP), which threatens its efficacy in east and southern Africa.
The results from the IMPROVE study were published in The Lancet in March 2023 and confirm that dihydroartemisinin-piperaquine (DP), a new antimalarial, is better tolerated, safer, and more effective in treating and preventing malaria infections in pregnancy than current WHO-recommended treatment (i.e. sulphadoxine-pyrimethamine, SP), but does not improve birth outcomes. IMPROVE is a multi-country clinical trial in western Kenya, northern Tanzania, and southern Malawi comparing the efficacy, safety and tolerance of monthly IPTp-SP (control) versus monthly IPTp-DP (new treatment), alone or combined with a single course of azithromycin at enrolment to reduce the adverse effects of malaria and curable STIs/RTIs in 4,680 pregnant women and their newborns in sub-Sahara Africa.
In view of the study findings, investigators in Uganda and Papua New Guinea are now examining to combine the potent non-malarial effects of SP on foetal growth with the superior antimalarial effects of DP, rather than replacing SP with DP in areas of high SP resistance. It is expected that WHO and countries in East and Southern Africa update their recommendation for preventing malaria in pregnancy, in line with the IMPROVE findings.
Implementing malaria prevention in infants (MULTIPLY study)
Intermittent Preventive Treatment of malaria in infants (IPTi) with sulfadoxine-pyrimethamine (SP) is a safe, affordable, and effective intervention for preventing malaria in infants which has shown to reduce rates of clinical malaria, anaemia and hospital admissions in children under one year of age by 30% and 21%, respectively. The WHO recommends its use for malaria prevention in this high-risk group since 2010, however, IPTi implementation has not been widely adopted in Africa, and to date, only Sierra Leone has adopted the IPTi intervention into policy and practice.
The MULTIPLY project aims to deliver the IPTi intervention in infants through the Expanded Program on Immunization (EPI) as a feasible, sustainable, equitable, and cost-effective strategy to prevent malaria in infants living in areas of moderate-to-high transmission in sub-Saharan Africa. The study is implemented in Mozambique, Togo and Sierra Leone which have different levels of SP resistance, malaria seasonality transmission patterns, different implementation of Seasonal Malaria Chemoprevention (SMC) programmes, and different implementation of IPTi as a national policy.
Around 45,000 children, 15,000 per country, are receiving up to six doses of IPTi for two years. If successful, it is expected that this intervention will be scaled up to include other regions/countries with moderate-to-high malaria transmission.
Post-discharge management of children with severe malarial anaemia (PMC-II study)
Severe anaemia is associated with significant post-discharge morbidity and mortality in children under five years, in particular, in malaria-prone areas. Recent studies have shown that giving three months of post-discharge malaria chemoprevention (PMC) with the long-acting dihydroartemisinin-piperaquine (DP) in transfused children with severe anaemia prevented 35% of deaths and reduced hospital re-admissions by six months. However, this benefit did not impede other non-malarial causes of readmissions among these children.
The PMC-II study combines DP with a wide-spectrum antibiotic, azithromycin (AZ), in order to collect data on whether the prophylactic intervention with DP plus AZ could be recommended as a cost-effective strategy for the post-discharge management of children with severe anaemia in malaria-endemic areas, and thereby to reduce the non-malarial causes of readmissions.
The study is enrolling 958 children in Kenya, Malawi and Uganda and following them for six months post-hospital discharge.
