World NTD Day 2024: investing to control and eliminate neglected tropical diseases
Global investment into research and development of new products for neglected tropical diseases (NTDs, also known as neglected infectious diseases) is limited and there is an urgent need to develop new or improved products and to optimise the use of existing products to achieve disease control and elimination. Neglected infectious diseases (NIDs) were added to the scope of the second EDCTP programme (EDCTP2) in 2014. Since then, our research funding has achieved important milestones that can help to control and eliminate these diseases.

EDCTP2 supports collaborative research studies that focus on the development of drugs, diagnostics and vaccines for NIDs. Capacity development is key towards sustainable programs and country ownership and EDCTP has integrated capacity development activities into all its research projects. In addition, EDCTP supports projects that facilitate the translation of research results into policy and practice, as well as providing fellowships for African researchers. In total, €80 million of EDCTP funding has been dedicated to reducing the burden of NID’s in sub-Saharan Africa by supporting 42 projects.
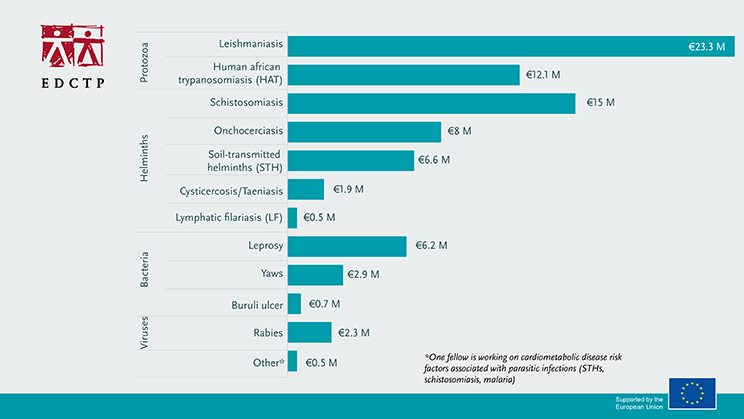
Further investments in NIDs will continue under the Global Health EDCTP3 programme. During COP28 meeting in December 2023, Global Health EDCTP3 pledged €46 million to tackle NIDs in sub-Saharan Africa. Since its launch in 2022, Global Health EDCTP3 has invested €19 million to support research in NIDs. An additional €22 million has been committed to a dedicated call for proposals to accelerate the development and integration of therapeutics against NTDs in sub-Saharan Africa launched on 18 January 2024.
Case study: Schistosomiasis treatment to preschool-aged children
An EDCTP-funded study contributed to the EMA recommendation of arpraziquantel, a key drug for control of schistosomiasis in young children
In December 2023, the European Medicines Agency (EMA) adopted a positive scientific opinion for arpraziquantel to treat schistosomiasis in preschool-aged children. The positive scientific opinion considered all supporting evidence, including the results of the pivotal phase III trial in Côte d’Ivoire and Kenya – which showed excellent efficacy of arpraziquantel, achieving cure rates of 90% or above, and was safe and well tolerated by young children affected by schistosomiasis. Led by the Paediatric Praziquantel Consortium, this trial was co-funded by EDCTP and the Global Health Innovative Technology (GHIT) through the PZQ4PSAC study.
In parallel with this regulatory work, the Consortium’s implementation research project, ADOPT, also co-funded by EDCTP and GHIT, is preparing for the introduction of arpraziquantel in the first endemic countries in Africa.
Case study: Improving treatment of acute sleeping sickness
A new treatment against a type of sleeping sickness mainly found in East Africa received EMA positive opinion.
In December 2023, the EMA adopted a positive scientific opinion of the use of Fexinidazole Winthrop as first oral treatment for T.b. rhodesiense sleeping sickness, an acute and lethal form of this parasitic disease found in Eastern and Southern Africa. The trial for treatment of T.b. rhodesiense with Fexinidazole Winthrop in Malawi and Uganda was supported by EDCTP through the HAT-r-ACC consortium.
This positive scientific opinion today paves the way for the update of World Health Organization (WHO) guidelines on sleeping sickness, as well as the extended indication and distribution of fexinidazole in African countries where T.b. rhodesiense is prevalent.
Case study: Better tools for control of parasitic worm infections
The STOP study is developing a convenient pill to improve control of parasitic worm infections in Africa.
The EDCTP-funded STOP consortium conducted a phase II/III trial of a fixed-dose combination drug of ivermectin and albendazole for the treatment of soil-transmitted helminths (STH) in children. This trial was completed in 2023 and the results of this study were positive. The results, together with other supporting evidence, were submitted in a dossier to EMA for review under the EU-M4all initiative (formerly article 58) in December 2023.
If EMA provides a positive scientific opinion, this combination of albendazole and ivermectin fixed-dose combination would be a valuable additional drug option for the control of STHs, especially for those species where drug efficacy is low, such as recently reported results from Ethiopia in a study done by the EDCTP-funded PROFORMA consortium.
Case study: Eliminating sleeping sickness in Côte d’Ivoire
The DiTECT-HAT project has made key contributions to the WHO-certified elimination of human African trypanosomiasis (HAT) in Côte d’Ivoire.
In 2021, WHO verified elimination in Côte d’Ivoire, an achievement that drew heavily on the work of the EDCTP-funded DiTECT-HAT project. The project has been evaluating three point-of-care rapid diagnostic tests and comparing results with laboratory-based methods, at sites in the Democratic Republic of the Congo, Côte d’Ivoire and Guinea. The Côte d’Ivoire studies identified tests suitable for use in the country, as well as symptoms associated with positive test results, which could act as a trigger for testing for HAT.
The project has also been assessing a potential approach for post-elimination monitoring, which will require testing on a population scale.
Côte d’Ivoire’s submission to WHO included DiTECT-HAT’s passive case-detection activities. As well its contribution to Côte d’Ivoire’s success in 2021, the DiTECT-HAT project’s work will also be highly relevant to other countries targeting HAT elimination.
Fellow profile: Professor Richard Phillips (Ghana)

EDCTP Fellow Professor Richard Phillips has established an extensive programme of research on Buruli ulcer in Ghana, in partnership with collaborators in Europe. His research has provided significant input into WHO-recommended treatments for Buruli ulcer. We asked Professor Phillips how his EDCTP Fellowship is helping advance his research to shorten the treatment of Buruli ulcer.
