Second largest public funder of TB research
In the 2021 Report on TB Research Funding Trends, an annual publication of Treatment Action Group, EDCTP was identified as the second largest public funder of TB research and the third largest funder of TB research overall. Between 2019 and 2020, our investments in TB research doubled from EUR 24 million to EUR 51 million, a tangible effort to invest in ending TB and saving lives.
In the summary of the report, where TAG outlines the pathway the EU can take to scale up support for TB R&D, it states: “A comparison of funding from 2019 and 2020 shows an increase in overall investments in spite of the economic shocks of the COVID-19 pandemic. However, this increase is due primarily to a doubling of spending by the European & Developing Countries Clinical Trials Partnership (EDCTP); like in the United Kingdom, investments from many other European countries in 2020 decreased.”
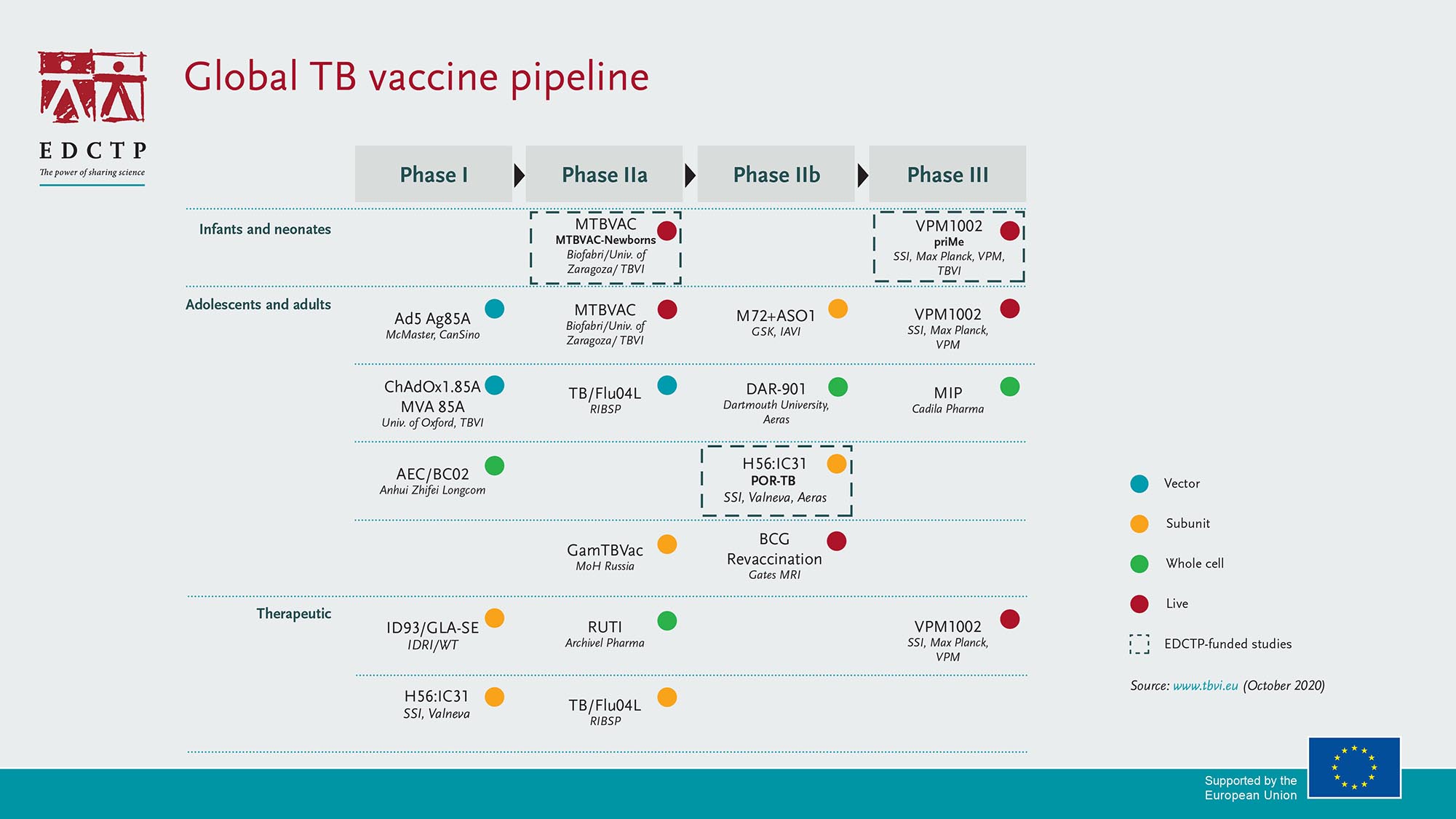
Overcoming key barriers to TB vaccine R&D and implementation

In addition to its investments in TB, EDCTP and the Amsterdam Institute for Global Health and Development (AIGHD) launched the Global TB vaccine R&D roadmap, also published in The Lancet. It identifies priorities for the development and implementation of new TB vaccines with the aim to coordinate and accelerate global action. The project was carried out in close collaboration with the World Health Organization.
The consultation led to the identification of three priorities: 1) diversify the pipeline; 2) accelerate clinical development; and 3) ensuring public health impact. Three cross-cutting enabling factors were also identified: funding, open science and stakeholder engagement.

“An effective vaccine against tuberculosis is an attainable goal if we invest much more in discovery and clinical research, use that funding in a clever way, share our findings and specimens, and already think now how such a vaccine should be delivered to reach maximum health impact. The unprecedented acceleration of vaccine development we have seen for COVID-19 should be the new normal – let’s not settle for less.”
Frank Cobelens, Amsterdam Institute for Global Health and Development, Lead author of the TB vaccine R&D Roadmap
EDCTP is also collaborating with WHO on Evidence Considerations for Vaccine Policy (ECVP) to reduce delays between vaccine recommendation and use.
TB vaccines: funding and coordination
EDCTP has awarded €51.18 million to support the development of TB vaccines through four collaborative research projects supporting vulnerable populations – newborn infants and adolescents: