World Tuberculosis Day 2015
Tuberculosis (TB) remains an epidemic in much of the world, causing the deaths of nearly one-and-a-half million people in 2013, mostly in developing countries. Every year approximately 9 million people fall ill with TB. A third of them, 3 million are not reached and consequently not treated. Unmistakably, tremendous progress has been made in recent years, and the world is on track to meet the Millennium Development Goal of reversing the spread of TB by 2015. But it is not enough. Drug-sensitive and drug-resistant TB constitute a global health security threat.
In May 2014, at the World Health Assembly, governments agreed on an ambitious new 20-year (2016-2035) strategy to end the global TB epidemic. According to the Stop TB Partnership “there is a critical need to address weaknesses in countries’ health systems, sustained and predictable funding, political engagement and support. We need countries to step up their domestic investments in TB in a cost efficient manner – in prioritized interventions that work and show impact. We need communities, people affected and civil society in the driving seat”.
Tuberculosis research and EDCTP
On the research side, there is dire need for more effective drugs with shorter and patient-suitable therapeutic regimens, better vaccines, and improved diagnostic tests for TB. Both multi-drug resistant TB (MDR-TB) and extensively drug resistant TB (XDR-TB) need to be addressed.
Together with other public and private funders, EDCTP – within the scope of its mandate – aims to address these problems. Its TB portfolio reflects this presenting a diverse range of diagnostics studies as well as treatment and vaccine trials.
TB research received the largest share of the funding in the project portfolio of EDCTP’s first programme (EDCTP1), with a total of € 70.68 million (33.3%) for 36 projects, with an additional 3.4% of grant funding (€ 7.23 million) for 12 projects on HIV/TB co-infection.

The EDCTP1 funding strategy for TB research has focused on the key issues and challenges in sub-Saharan Africa, recognising the impact of HIV-TB infection in this region. An EDCTP-commissioned bibliometric analysis has tracked the increasing prominence of this region in TB and HIV/TB research, showing that TB research output in sub-Saharan Africa increased by 81% between 2003 and 2011. EDCTP-funded TB and HIV/TB publications had an exceptionally high citation impact (NCI: 4.08) that was more than four-times the world average.
Of note, EDCTP-funded research papers on HIV/TB co-infections were cited more than five-times more often than the world average (5.1). With the release in 2014 of several major publications from EDCTP-funded trials, the high citation impact of EDCTP-funded research on TB is expected to continue.
TB-research funding under EDCTP's second programme
The second EDCTP programme (EDCTP2) includes funding clinical research on TB as one of the major poverty-related disease in sub-Saharan Africa. However, the nature of its calls for proposals has changed as compared to the first programme (EDCTP1).
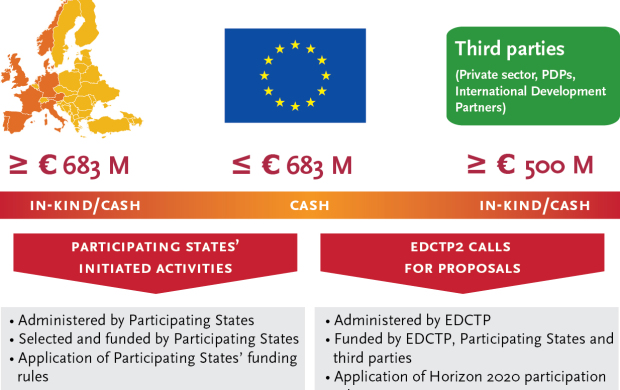
Whereas EDCTP1 launched mainly disease specific calls, EDCTP2 calls for proposal are broader and apply to all diseases within the scope of EDCTP, namely HIV/AIDs, TB, malaria and other poverty-related and neglected infectious diseases in sub-Saharan Africa. This approach creates more opportunities for funding for TB clinical research applications.
The European Union provides a contribution of up to €683 million for the EDCTP2 programme, matching the €683 million commitment of the European countries participating in EDCTP2. Additionally, one of the EDCTP2 objectives is to leverage €500 million from third parties. EDCTP publishes annual work plans which contain details on the activities to be funded including the calls for proposals.
Funding schemes
Three distinct types of funding schemes or ‘actions’ are supported under the EDCTP programme: Research & Innovation Actions (RIA), Coordination & Support Actions (CSA), and Training & Mobility Actions (TMA).
Research & Innovation Actions (RIAs)
Actions primarily consisting of clinical research activities and clinical trials in partnership with SSA aiming at increasing the number of new or improved medical interventions for HIV/AIDS, tuberculosis, malaria and other poverty-related diseases, including neglected ones, in particular in SSA.
Coordination & Support Actions (CSAs)
Actions primarily consisting of accompanying measures, such as activities to develop, strengthen and extend clinical research capacities in SSA. Actions may also involve targeted measures to maximise the public health impact of research results by promoting their translation and supporting their uptake in policy-making, health systems and clinical practice at local, national and/or international level. In particular, CSAs will support SSA countries in developing a robust ethical and regulatory framework for conducting clinical trials.
Training & Mobility Actions (TMAs)
Actions primarily consisting of activities fostering career development (fellowships) of individual junior and senior researchers from SSA, supporting training and mentorship of researchers, and promoting mobility of individual researchers and research staff.
Video on EDCTP-funded research: TB-IRIS in the PredART-study
On the occasion of World TB day 2015, EDCTP presents a new project video on the PredArt study, coordinated by Dr Graeme Meintjes (University of Cape Town, South Africa). The study is conducted in an HIV-TB clinic in Khayelitsha, a community of 500,000 people on the outskirts of Cape Town with very high rates of TB and HIV.
The PredART study aims to develop a treatment intervention to address the TB-associated immune reconstitution inflammatory syndrome (TB-IRIS) complication in HIV-TB co-infected patients. When patients have both HIV and TB infections, treatments need to be combined. One of the most common complications in those patients is TB IRIS. As the successful start of HIV-treatment with antiretrovirals leads to a rapid reconstitution of the immune system, a strong inflammatory response to the tuberculosis and other infections may result. TB IRIS can be life threatening or at the very least undermine patients’ confidence in their treatment. Currently there is no preventative strategy for TB IRIS, therefore the PredART study is testing an intervention with prednisone, which – if successful – will inform international guidelines for the treatment of HIV-TB co-infection.
More information
