World AIDS Day 2018: Supporting R&D partnerships to tackle HIV
World AIDS Day 2018 marks its 30th anniversary. Significant progress has been made in the fight against the disease during these three decades. However, the Global AIDS Update 2018 report shows that much needs to be done to reach the 2020 targets. According to the report, ‘new HIV infections are rising, HIV-related deaths are not falling fast enough and flat resources are threatening success’.
Over the last 15 years, EDCTP has achieved significant progress in bringing together researchers and institutions in Europe and Africa to advance medical interventions against HIV. EDCTP-funded studies on HIV have made vital contributions to the development of antiretroviral drug formulations tailored to children and facilitating their widespread introduction in Africa. Other studies were carried out in the prevention of mother-to-child transmission of HIV and in the detection and treatment of opportunistic fungal infections, which are responsible for one in five HIV-related deaths.
“Looking back, we are encouraged seeing the tangible achievements made in advancing medical interventions against HIV. We are hopeful to see even more breakthroughs as we scale up our efforts. We embrace more collaborative research and development approaches across diseases and disciplines and also foster a community/patient-centred and innovative environment.”
Dr Michael Makanga, EDCTP Executive Director
Investments in HIV research 2003-2018
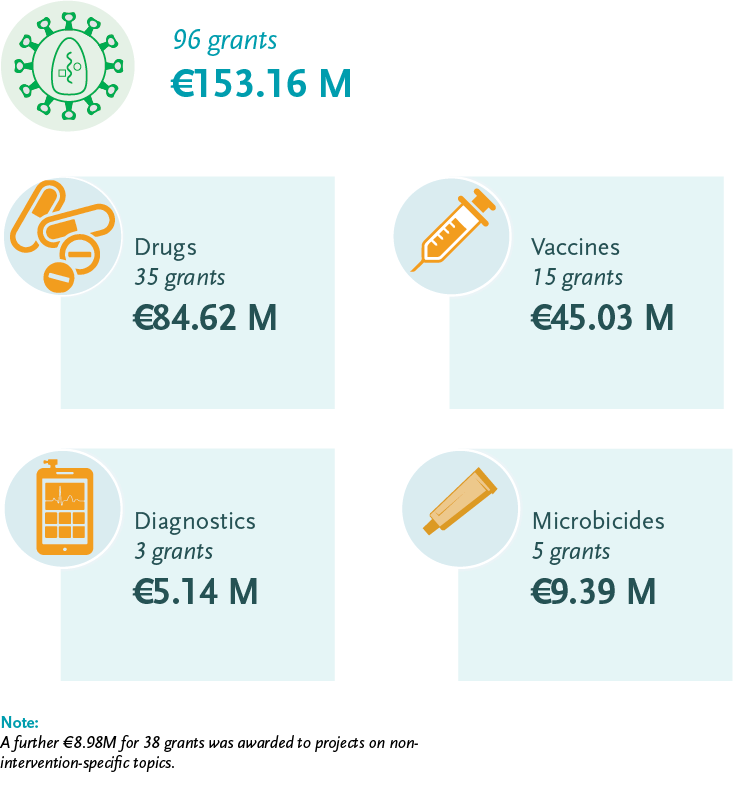
EDCTP HIV portfolio 2014-2018
EDCTP’s strategic research agenda addresses treatment, prevention, and product-focused implementation research with prioritisation of the following needs:
- Optimised treatment regimens and drug formulations for key groups such as children, pregnant women and adults with co-infections and co-morbidities;
- Increased access to proven interventions; and
- Novel HIV prevention strategies such as pre-exposure prophylaxis and vaccines.
New back-up treatments for children with HIV
The CHAPAS-4 study is evaluating a suite of new therapies for children with drug-resistant HIV infections. The long-standing CHAPAS consortium has developed world-leading expertise in HIV-related clinical trials in African children. Its previous studies (CHAPAS 1, 2 and 3) have been instrumental in enhancing children’s access to antiretroviral therapy in Africa. Its latest trial, CHAPAS-4, is evaluating a range of new drug combinations for second-line HIV therapy. It will provide key data on optimal treatments for HIV infections in children. The trial will also investigate how metabolism of the drugs is affected by anti-TB therapies – many children in sub-Saharan Africa are likely to be infected with both HIV and TB. In the absence of third-line therapies, it is essential that second-line therapies are used as wisely as possible to prevent the emergence of essentially untreatable HIV infections in individuals who will need lifelong treatment.
Finding ‘hidden’ HIV-infected infants
The PROMISE-EPI study is evaluating a novel strategy for identifying infants who, despite big drops in mother-to-child transmission, have still been infected with HIV. For more than a decade, the PROMISE consortium, set up with EDCTP funding, has developed a portfolio of studies addressing
mother-to-child transmission of HIV in Africa. Having generated vital evidence on the importance of pre-emptively treating infants to prevent transmission of HIV*, the latest study could identify a readily implementable strategy for reducing still further infant infection rates, and for identifying those who nonetheless have been infected.
Combining drugs and vaccines to prevent HIV infection
The PrEPVacc trial is the first to test whether a combination of pre-exposure prophylaxis and an experimental vaccine can prevent HIV infection. PrEPVacc will generate the first evidence of the ability of vaccine plus PrEP combinations to prevent HIV infections – information that would have major public health importance. It will also provide key data on the ability of the experimental vaccines to stimulate protective immune responses, and the likely success of DNA plus protein prime-boost vaccine strategies. Data on the nature of the immune responses triggered by the vaccines may also reveal which are key to the prevention of HIV infection.
Speeding up HIV treatment in infants
The LIFE study is assessing whether use of new rapid diagnostic tests for HIV leads to quicker and better treatment of infants with HIV. It will fill important gaps in the evidence base. It will reveal whether use of rapid point-of-care HIV diagnostics at birth increases antiretroviral therapy coverage and benefits infant survival; whether measurement of virus levels in mothers at delivery leads to the adoption of special measures to prevent mother-to-child transmission; and what the financial and practical implications are of rapid diagnostic use. The study will, therefore, provide key data for decision makers on the cost-effectiveness and practicalities of implementing the new technology and new models of care.
More information on the EDCTP portfolio
The EDCTP2 programme is supported under the Horizon 2020, the European Union’s Framework Programme for Research and Innovation.
Tackling infectious disease in sub-Saharan Africa: EDCTP-funded clinical studies for medical interventions 2003-2018 provides an overview of EDCTP-funded clinical studies for medical interventions against poverty-related infectious diseases, 2003-2018.
EDCTP’s public Project Portal provides an up-to-date view on the projects supported as our portfolio grows and gives details of each project’s aims and objectives. Search “HIV” or “Co-infections” in the “classification” box to see current HIV projects.
The portfolio of projects supported under the first EDCTP programme can be viewed here.
