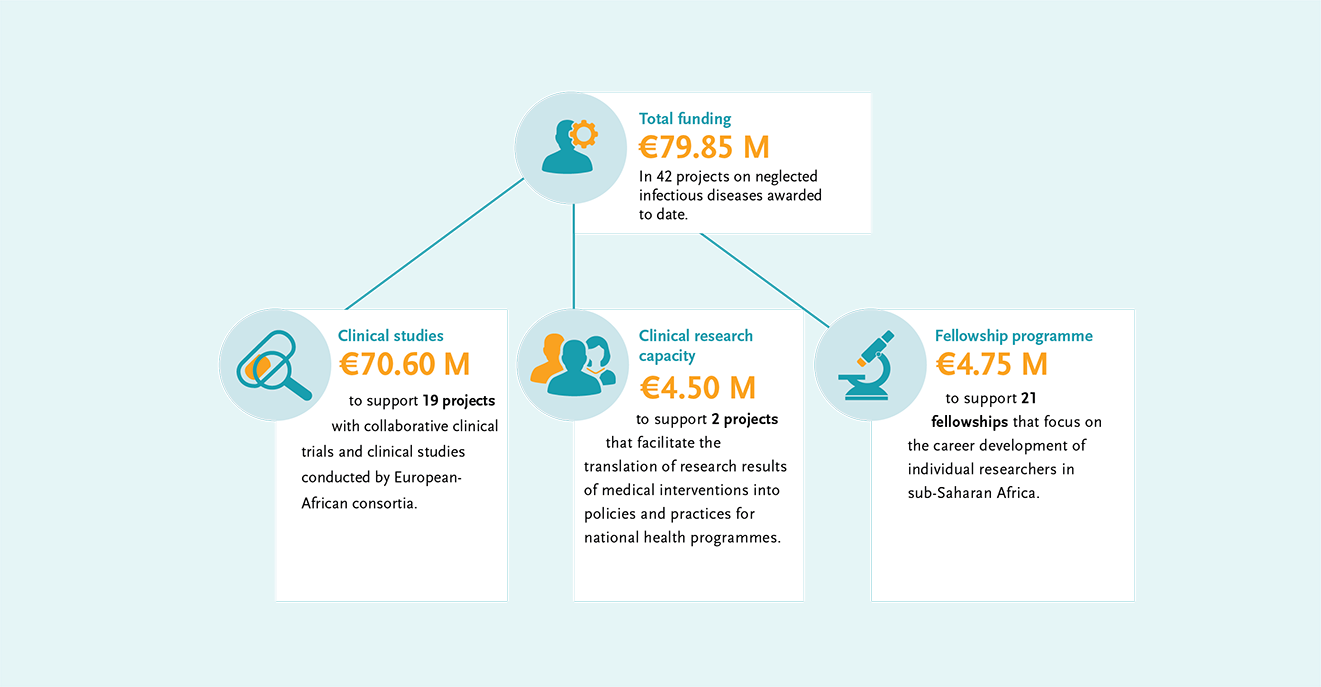
In addition, EDCTP supports projects that facilitate the translation of research results into policy and practice, as well as fellowships for African researchers. In total, €79.85 M of EDCTP funding has been dedicated to reducing the burden of NID’s in sub-Saharan Africa.
World NTD Day 2022 – EDCTP remains 100% committed to ending neglected tropical diseases
In another year of changes in the funding landscape, EDCTP has stayed 100% committed to ending neglected infectious diseases. Global investment into research and development of new products for these diseases is limited and there is an urgent need to develop new or improved products and to optimise the use of existing products to achieve disease elimination. In 2014, EDCTP added neglected infectious diseases to the scope of its second programme. Our research funding focuses on protecting the most vulnerable groups in society such as infants, children, adolescents, pregnant women and individuals with co-infections and co-morbidities and ensuring that they benefit from new medical interventions. We continue to support projects that have experienced setbacks due to the COVID-19 pandemic, and celebrate the projects that have already achieved promising results.
“Amidst competing priorities of diseases of epidemic potential, EDCTP maintains commitment to neglected infectious diseases and populations. We are greatly encouraged by the growing and promising studies evaluating new medicines and combinations, as well as the research on optimising and managing co-endemic infections involving NTD’s and related capacity building.”
Dr Michael Makanga, EDCTP Executive Director
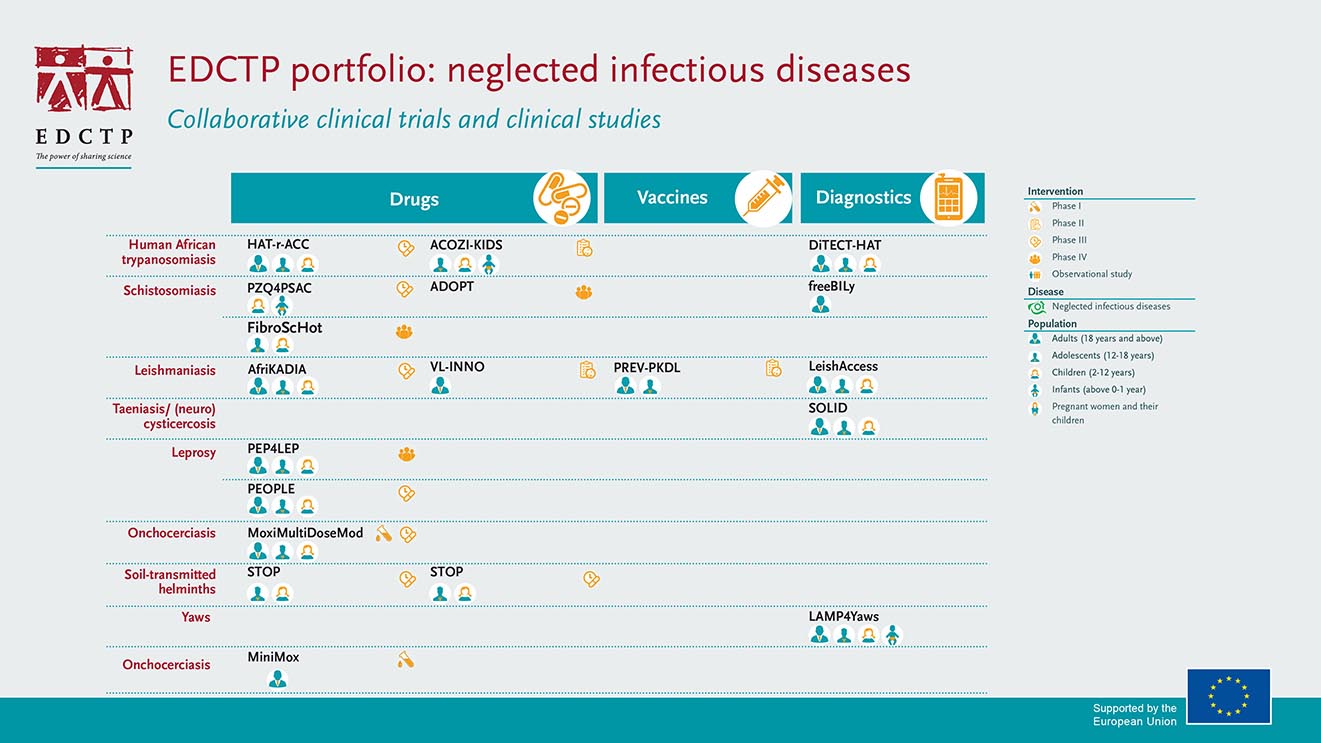
EDCTP portfolio and investments
EDCTP’s collaborative clinical trials and clinical research portfolio on neglected infectious diseases (NIDs) has major investments in therapeutic development and reformulation. Projects cover the key neglected infectious diseases affecting sub-Saharan Africa, including schistosomiasis, leishmaniasis, onchocerciasis, Human African Trypanosomiasis and leprosy. There is a focus on the development of tools to facilitate disease elimination, including new formulations of drugs for young children and diagnostics to support the path to elimination. EDCTP has invested €70.6 million in 19 clinical projects on NIDs in sub-Saharan Africa.
Case study: Paediatric schistosomiasis drug arpraziquantel completes Phase III study
Schistosomiasis (also known as bilharzia) is one of the most prevalent parasitic diseases. Left untreated, schistosomiasis can lead to anemia, stunted growth, reduced learning ability and chronic inflammation of the organs, which can be fatal in the most serious cases. An estimated 56 million girls and women are affected by Female Genital Schistosomiasis which damages the reproductive organs. As efforts focus on morbidity control and elimination, there is a pressing need to treat preschool-age children.
The Pediatric Praziquantel Consortium has developed arpraziquantel, a new pediatric medication to treat schistosomiasis in preschool-aged children. In 2021 the Consortium announced the completion of its pivotal Phase III trial in Côte d’Ivoire and Kenya. The results of the trial, co-funded by the Global Health Innovative Technology (GHIT) Fund and EDCTP and sponsored by Merck, confirm a favourable efficacy and safety profile for arpraziquantel in children 3 months to 6 years of age, affected by schistosomiasis. The Consortium aims to submit the regulatory file to the European Medicines Agency (EMA) in 2022 under the EU-M4all procedure for high-priority medicines for markets outside the European Union.
The Consortium has been awarded a second EDCTP grant to research the most effective ways to introduce and roll out the new medication in affected communities in Côte d’Ivoire and Kenya.
Case study: Afrikadia completes phase III study of oral miltefosine (MF) and paromomycin (PM) to treat visceral leishmaniasis
AfriKADIA is a consortium of ten organisations working to find improved treatments and diagnostic tools for visceral leishmaniasis in eastern Africa. The Consortium has now completed a large-scale Phase III clinical trial to assess the efficacy and safety of a combination of oral miltefosine (MF) and paromomycin (PM) in treating visceral leishmaniasis. The current combination treatment used to treat visceral leishmaniasis, although effective, remains sub-optimal as patients may suffer from rare but major side effects, such as cardiotoxicity, hepatotoxicity, and pancreatitis. The treatment is also difficult to administer, since patients must be hospitalised and endure two painful injections every day for 17 days.
If found to be efficacious, the introduction of MF (which has been an effective tool in the elimination toolbox in South Asia for over 15 years) in combination with PM will reduce both the difficulty in administering treatment and the length of hospital stays for patients. The clinical trial also assessed innovative, non-invasive diagnostic tools for the detection and management of visceral leishmaniasis cases in routine patient care.
Read DNDi‘s story ‘We are building a generation of scientists who will transform research in Africa’ (with Dr Michael Makanga)
Fellows in times of COVID-19: Dr Michael Frimpong
Dr Michael Frimpong is a senior scientist at the Kumasi Centre for Collaborative Research (KCCR), and a lecturer at the Kwame Nkrumah University of Science and Technology (KNUST), Ghana. Dr Frimpong was awarded an EDCTP Career Development Fellowship for his research on rapid detection of Buruli ulcer. He explores the potential of recombinase polymerase amplification (RPA) as a tool for the rapid diagnosis of Mycobacterium ulcerans infections. As so many researchers throughout the African continent, Dr Frimpong’s work was challenged when the COVID-19 pandemic hit. In this video, which was created late 2020, Dr Frimpong shows us how his team repurposed a mobile testing lab to reach communities in Ghana that didn’t have access to COVID-19 testing.
We followed up with Dr Frimpong and asked how his work was affected by the COVID-19 pandemic:
Dr Frimpong, how was you research into buruli ulcer affected by the COVID-19 pandemic?
“The lockdowns and movement restrictions meant that for several months we could not visit our Buruli ulcer clinics where we recruit participants for our studies. Most of these buruli ulcer care centres were converted to COVID-19 treatment centres and healthcare workers who assist our projects were reassigned mainly focused on COVID-19 patients care and contact tracing. Even though the buruli ulcer care did not stop as a result of the pandemic, participants were reluctant coming to these clinics for fear of contracting COVID-19. This meant that new projects could not be initiated and ongoing projects had to be suspended over a period. It also meant that people who were infected by buruli ulcer over that period did not receive immediate care and reported later to the clinics with several forms of the disease defeating our control strategy of early detection, treatment and cure.
How did you apply your expertise in NID’s in those first months of the pandemic?
“My experience in molecular diagnosis for NID’s was used to support my country’s response against COVID-19 mostly in diagnostics. The mobile suitcase laboratory platform that we have developed for buruli ulcer detection in remote settings was repurposed for COVID-19 detection in a mobile van laboratory for confirmation of new infections and disease surveillance in hotspots and remote communities far away from reference laboratories. We used this model to set up two laboratories which have become major testing centres in Ghana bringing testing closer to the people.”
Were you able to resume your research into buruli ulcer when the pandemic hit?
“My work on Buruli ulcer was briefly halted during the early part of the pandemic due to the lockdowns and movement restrictions, but resumed once the lockdown was lifted. I successfully completed my EDCTP project (BU-RPA) and I am currently working on a new microbiome project on Buruli ulcer lesions to understand the effect of the microbial community on treatment response. We are also looking for new funding to conduct an independent evaluation of the diagnostic tool we developed for Buruli ulcer during EDCTP fellowship in several endemic countries in Africa.”
EDCTP Outstanding Female Scientists Award: Professor Margaret Gyapong
In October 2021, Professor Margaret Gyapong received the EDCTP Outstanding Female Scientist Award for her scientific contribution and measurable impactful research capacity. She is a true ambassador to achieving equity in research for health, and has worked relentlessly to improve access to tools that reduce the burden of malaria and neglected tropical diseases. Her team strives to understand the potential bottlenecks the health system faces in the introduction of new or improved tools (vaccines, new treatment regimens). Working with the health system, Professor Gyapong explores these bottlenecks so that feasible solutions may be found.
In 2017, she was one of twelve women honoured by women in global health to receive the first ever Heroines of Health award for her work drawing attention to the needs of women suffering from the consequences of neglected tropical diseases. Professor Gyapong’s ground‐breaking work in this field has driven much of our understanding of the gendered effects of NTDs, and female genital schistosomiasis.
About World NTD Day
On 12 November 2020, the World Health Organization (WHO) officially endorsed the new Roadmap to fight neglected tropical diseases (NTD) and end NTDs as a public health burden by 2030. The roadmap sets global targets and milestones to prevent, control, eliminate and eradicate 20 neglected tropical diseases (NTDs) and disease groups. The main global target for 2030 is to reduce the number of people requiring treatment for NTDs by 90%. Another goal is for at least 100 countries to have eliminated at least one NTD in 2030 – linking to this year’s World NTD Day ‘100 % Committed’ movement.


