World TB Day: the promise of ending the TB epidemic
World TB Day puts the spotlight on tuberculosis (TB), a poverty-related infectious disease of epidemic proportions. Currently, it causes more deaths than any other infectious disease, mostly in low- and middle-income countries. Every year, an estimated 10 million people fall ill; in 2019, 1.4 million people died from TB. All members of the United Nations committed to ending TB as an epidemic by 2030. However, the 2020 WHO progress report concluded that the world is not on schedule to achieve the 2022 targets. The COVID-19 pandemic even threatens to reverse recent progress towards global TB targets.
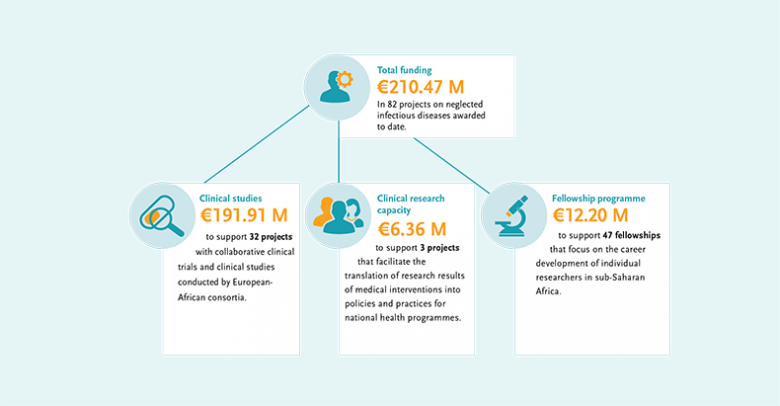
Chronic global underfunding of TB research means that cheap, accessible point-of-care tests, better vaccines and simple, short treatment regimens are lacking. Ending TB depends on the development and rapid uptake of new tools and innovation
“Our steady commitment to fight TB has led to a growing R&D portfolio of grants on novel diagnostics, more patient-friendly treatments, and experimental vaccines for pre-and post-exposure use – especially targeting vulnerable populations. Moreover, we have invested in international coordination of TB vaccine R&D, and preparation of ground for a new global TB vaccine roadmap. EDCTP is convinced a concerted global R&D effort will end TB.”
Dr Michael Makanga, EDCTP Executive Director
The TREATS study
The HPTN (PopART) trial was the largest trial ever undertaken of a combined HIV and TB intervention, involving around one million people. It found that targeting entire populations in a test-and-treat strategy significantly cut the number of new HIV infections. The EDCTP-funded TREATS study is using the unique infrastructure provided by the PopART trial to determine whether similar impacts can be achieved for TB.
By end of 2020, the TREATS study completed the majority of its follow-up visits. Focusing on 14 communities in South Africa and Zambia, the project collated PopART data on population-level TB notification, the prevalence of TB, and the incidence of TB disease and TB infection. More than 4,500 young people aged 15–25 were recruited and followed up to determine whether the PopART intervention reduced TB infection.
New diagnostic tools, including molecular Xpert Ultra and computer-aided analysis of digital chest X-rays, are being tested to determine their utility. Qualitative research on the impact of the intervention has found that people diagnosed with TB often experience significant social isolation that can delay treatment-seeking and lead to mental health issues.
The results of the TREATS study should reveal the impact of the PopART intervention on the TB disease burden, as well as its overall cost-effectiveness. The demonstration of benefits for TB as well as HIV control would provide further evidence to encourage a wider take up of test-and-treat strategies for HIV and TB.

Colliding epidemics: HIV, TB and COVID-19
Taking advantage of the infrastructure provided by the EDCTP-funded TREATS project, the TREATS-COVID project – funded by EDCTP under its 2020 COVID-19 emergency call – is seeking to understand the spread of COVID-19 in an urban population of Zambia. The project is working closely with a community of around 28,000 people that have been involved in research studies for many years.
All households are being visited, and COVID-19 cases and their household contacts are being followed up. All people of 15 years and above will be screened for symptoms of COVID-19. A second study will test 4000 people for past infection, as well as for HIV and TB infection.
Reliable and convenient tests are essential for monitoring the COVID-19 epidemic. The project will evaluate a range of potential community-based point-of-care tests to detect SARS-CoV-2 and antibodies to the virus. These will include a novel computer-aided diagnostic tool for interpretation of chest X-rays, developed by one of the project partners, initially for TB but since adapted for COVID-19.
The project team will use the information collected to develop models of COVID-19 transmission that better reflect the local situation, and extrapolate them to wider populations.
Diagnostics: TriageTB
The TriageTB study aims to refine and prospectively evaluate the performance of a point-of-care multi-biomarker test (MBT) as a laboratory-free triage test on fingerstick blood in three countries, Uganda, The Gambia and South Africa. An effective triage test for active TB could significantly speed up and streamline diagnostic approaches in resource-limited settings. The trial has begun recruiting at the end of 2020, adapting its procedures to implement rigorous COVID-19 prevention measures to ensure the safety of both patients and staff.
This first recruitment phase will be completed at the end of April 2021. The findings from this phase will enable the development of the signature to be used in the point-of-care test that will be validated in the second recruitment phase. (More information in the TriageTB news item.)
TB vaccines
Prevention of infection has relied on the BCG vaccine, which is only partially effective and has multiple drawbacks. Now, however, innovative new approaches are being used to create more powerful vaccines with greater potential to prevent infection with Mycobacterium tuberculosis (Mtb).
EDCTP is funding four TB vaccine trials. The MTBVAC-Newborns (to be followed by MTBVACN3) and priMe projects are focusing on novel vaccine alternatives to BCG to prevent infection in infants. By contrast, the POR-TB project is aiming to prevent the recurrence of disease in patients supposedly cured of Mtb by antibiotic treatment – typically, one in ten patients will relapse.
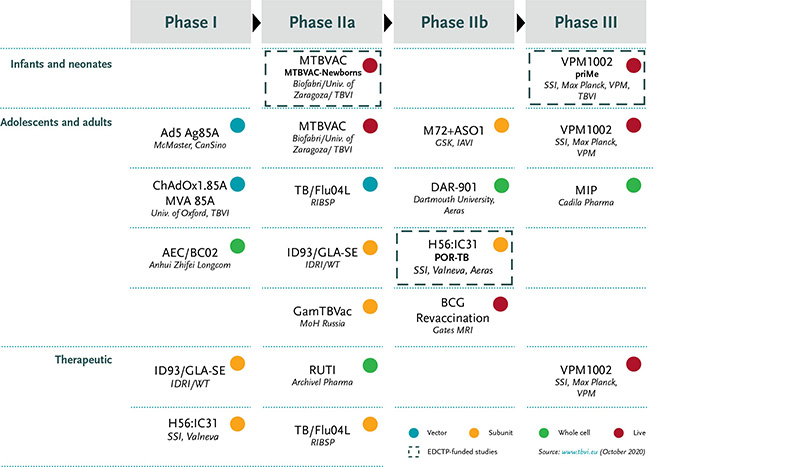
MTBVAC-Newborns
The MTBVAC-Newborns study is carrying out a phase II clinical trial in newborn infants in South Africa to evaluate the safety of increasing doses of the candidate TB vaccine MTBVAC and the strength of anti-TB immune responses, in comparison with BCG. In parallel, the consortium is building capacity for future large-scale trials in Senegal and Madagascar, which have a high burden of TB.
The South African Tuberculosis Vaccine Initiative (SATVI) conducted this study in the Western Cape of South Africa, recruiting women in the third trimester of pregnancy, following them through delivery before enrolling their newborns. Babies are followed up over a period of 12 months. Recruitment was conducted over two years and completed in March 2021 with a total of 99 healthy, BCG-naïve, HIV-unexposed newborns included in the trial. The completion of the enrolment is especially celebrated as 2020 has proved to be a very challenging year due to the COVID-19 pandemic and the lockdown in South Africa.
The phase III efficacy study (MTBVACN3) is planned to start in 2022 with the MTBVAC vaccination of nearly 7,000 infants. EDCTP has awarded a grant of more than 19 million euros to co-fund this pivotal phase III trial. (See also the TBVI news item.)
More information
- WHO – World TB Day 2021 ‘The Clock is Ticking’
- WHO – Global Tuberculosis Report 2020
- WHO – Summary of the report: Progress towards achieving global tuberculosis targets and implementation of the UN Political Declaration on Tuberculosis
- TB Alliance: Amid Global Pandemic, TB Treatment Innovation Advances at an Unprecedented Pace
- EDCTP video (2020) highlighting three EDCTP tuberculosis projects: PanACEA (drug development to shorten TB treatment); Predict-TB (new diagnostics for shortened therapy); and POR-TB (new vaccines for prevention).
- PANDORA-ID-NET press release on a 2021 theme issue on TB of the International Journal of Infectious Diseases. The series of 18 articles are written by global authorship of over 150 authors from all continents, including collaborators from EDCTP-funded projects PANDORA-ID-NET, CANTAM and EACCR.
PANDORA-ID-NET dons lead Special TB Theme Issue of International Journal of Infectious Diseases, 24 March 2021.
